Fine Needle Aspiration Cytology - Other Body Sites
Other Body Sites
Michele M. Weir, MD, FRCP
Introduction
This section deals with fine needle aspiration biopsies (FNAB) from kidney, adrenal gland, gonads, bone and soft tissue.
Kidney FNAB
FNAB of a renal mass is an uncommon procedure, since most patients with a renal mass go directly to resection. However, FNAB does play a role in the evaluation of a renal mass when:
- the imaging features are indeterminant for classification;
- the patient is not a surgical candidate; and
- surgical planning is required (kidney or ureter sparing surgery)
FNAB of renal tumors is reported to have a sensitivity of 79-92% a specificity of 92-99% and an overall diagnostic accuracy of 73-95%. Due to tumor heterogeneity and the importance of cytogenetic classification, definitive diagnosis for some renal neoplasms is best rendered on the histologic specimen. Some Hale’s colloidal iron negative oncocytic neoplasms may be difficult to further subtype on FNAB, and only a differential diagnosis should be rendered as a result.
In adults, the most common non-neoplastic cause of a renal mass is a renal cyst. Other lesions may include an abscess and xanthogranulomatous pyelonephritis. Among the renal neoplasms, the most common benign entity is oncocytoma and the most common malignant entity is a renal cell carcinoma (RCC). Other neoplasms include benign angiomyolipma and renal pelvis urothelial carcinoma.
Cytology of Kidney FNAB
- Normal
- Low Cellularity Sample
- Isolated Cells, or Small Groups
- Glomeruli:
- capillary loops, spindled and round cells
- no atypia, no spherules or papillae (unlike papillary RCC)
- cellular globular structures
- mimic: papillary RCC
- Proximal Convoluted Tubule (PCT):
- tubules and sheets
- abundant granular cytoplasm
- ill-defined fragile cytoplasm without cell borders (unlike oncocytoma)
- bland nucleus, prominent nucleolus
- mimics: oncocytoma, RCC
- Distal Convoluted Tubule/collecting Duct (DCT/CD):
- tubules, flat sheets (unlike RCC)
- well-defined cytoplasm, smaller cells
- no vacuoles (unlike RCC)
- no nucleolus
- mimic: RCC
- Oncocytoma
- Clean Background
- Dyshesive Single Cells or Loose Clusters, No Stripped Nuclei
- Rarely in Large Groups (Unlike RCC)
- Small Uniform Nuclei, Smooth Borders (Unlike RCC)
- Focal Nuclear Atypia, Binucleation, Inconspicuous Nucleoli
- Abundant Uniformly Granular Well-defined Cytoplasm
- No Vacuoles (Unlike RCC)
- Sharp Well Defined Cell Border (Unlike PCT Cells)
- Vimentin Negative, Cytokeratin 8/18 Positive (Use Biotin Block)
- Hale’s Colloidal Iron Negative, or Perinuclear/atypical Staining Present
- Electron Microscopy: Mitochondria
- MIMICS: PCT, Chromophobe RCC, Conventional RCC with Granular Cytoplasm
- Renal Cell Carcinoma
- Conventional/common/clear Cell Type (CRCC)
- clean or necrotic background
- cohesive monolayered sheets (unlike oncocytoma)
- prominent branching capillaries
- rare single cells (low grade) → more single cells and stripped nuclei (higher grades) (unlike oncocytoma)
- bland nuclei, no nucleoli (low grade)
- larger atypical nuclei, some bizarre, nucleoli prominent (higher grade), (unlike oncocytoma, chromophobe RCC)
- eccentric nucleus, extruded from cells
- more uniform nuclei than chromophobe RCC
- foamy vacuolated cytoplasm (unlike ONC and normal)
- clear, or granular (not uniform) abundant cytoplasm (low N/C ratio)
- intracytoplasmic Mallory-like bodies
- vimentin, cytokeratin positive (use biotin block)
- Hale’s colloidal iron negative
- electron microscopy: glycogen, lipid; mitochondria in some
- MIMICS: distal convoluted tubule and collecting duct, oncocytoma, chromophobe RCC
- Chromophobe Type
- clean background
- sheets, clusters, single cells (dyshesive, but less than CRCC)
- bare nuclei (unlike oncocytoma)
- more variation in cell & nuclear size (than oncocytoma, CRCC)
- vesicular nuclei, binucleation, inclusions
- irregular nuclear outline (unlike oncocytoma, CRCC)
- prominent nucleoli in some
- abundant granular cytoplasm
- perinuclear clearing, prominent cell borders (“koilocytic”)
- fluffy/clear/granular not uniform cytoplasm
- vimentin negative, cytokeratin positive (use biotin block)
- Hale’s colloidal iron positive – uniform, dense, cytoplasmic
- electron microscopy: microvesicles; mitochondria if eosinophilic variant
- MIMICS: oncocytoma, CRCC
- Conventional/common/clear Cell Type (CRCC)
Adrenal FNAB
FNAB of an adrenal mass is usually performed to confirm a metastasis, or in the work-up of an incidental nodule. A functional, or suspected pheochromocytoma is a contraindication for FNAB due to possible hypertensive crisis and/or death. FNAB of adrenal tumors is reported to have a sensitivity of 85-94% and for metastases, a specificity of 100%.
In adults, the most common neoplasm in the adrenal gland is a metastasis from lung, or breast. Other frequent sites of origin include the gastrointestinal tract, pancreas, kidney and skin (melanoma). Adrenal primary neoplasms include those of cortical origin (adenoma, carcinoma) and those of medullary origin (pheochromocytoma). Other primary adrenal lesions include myelolipoma, cysts and cortical nodular hyperplasia.
Cytology of Adrenal FNAB
- Normal
- Low Celularity
- Cortex: Outer Layers
- foamy lipid rich background
- single cells, clusters
- bland oval nuclei
- no or small nucleoli
- abundant vacuolated cytoplasm with frayed edges
- MIMICS: may be indistinguishable from benign adrenal cortical nodule, some adrenal cortical carcinomas
- Cortex: Inner Layer
- no vacuolation
- granular eosinophilic cytoplasm
- smaller cells
- ipofuscin pigment
- Medulla:
- basophilic cytoplasm
- large eccentric nucleus
- conspicuous nucleoli
- fine granular chromatin
- Benign adrenal cortical nodule
- May be Cellular Sample
- Foamy Lipid Rich Background (Absent in RCC)
- Cohesive Fragments with Sinusoidal Endothelial Cells
- Stripped Nuclei
- Round to Oval Nuclei (More Uniform Than RCC)
- No or Small Nucleolus
- Multinucleation
- Vacuolated Cytoplasm (More Than RCC)
- Vimentin Positive, Cytokeratin (Low Molecular Weight), Positive in Some
- Inhibin, Melan-A, Calretinin Positive
- EMA, CK7, CK20 Negative
- MIMICS: RCC, and May be Indistinguishable from Normal Adrenal Gland and Some Adrenal Cortical Carcinomas.
- Adrenal cortical carcinoma
- MIMICS Adenoma Features
- Necrosis May be Present
- May See Malignant Nuclear Criteria
- Histological Assessment Required to Distinguish Larger Adenomas from Carcinomas
- Similar Immunoprofile as Adenoma
- MIMICS: May be Indistinguishable from Normal Adrenal Gland and Adrenal Cortical Adenoma; Pheochromocytoma, Other Malignancies, if Poorly Differentiated
- Pheochromocytoma
- 3 Cell Types
- #1 – fibrillary cytoplasm, oval hyperchromatic nuclei – may show anisonucleosis/pleomorphism/binucleation
- spindle cells (sustenacular cells)
- plasmacytoid cells
- Melanin Pigment in Some
- Dyshesive, Single Cells, Some Clusters
- Prominent Variation in Nuclear Size & Shape
- Red Cytoplasmic Granularity on Air Dried Material
- Synaptophysin, Chromogranin Positive; S-100 Positive in Spindle Cells
- MIMICS: Adrenal Cortical Carcinoma, Other Poorly Differentiated Malignancies
- 3 Cell Types
- Metastatic carcinoma
- Uniform Cell Population
- Malignant Nuclear Criteria, May Have Neuroendocrine Features
- Glandular or Squamous Differentiation of the Cytoplasm May be Present
- Necrosis May be Present
- Cytokeratin Positive
- CK7, CK20 Profile May Narrow Site of Origin
- Usually Inhibin, Melan-A, Calretinin Negative
- TTF-1 May Aid in Lung Origin Confirmation
- MIMICS: Pheochromocytoma, Adrenal Cortical Carcinoma, Other Poorly Differentiated Malignancies
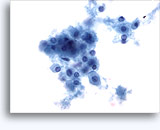
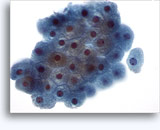
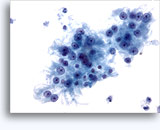
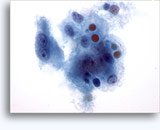
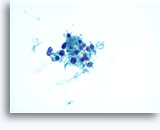
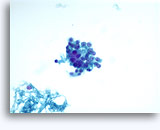
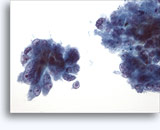
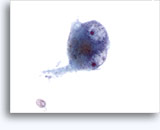
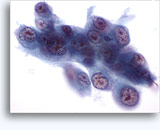
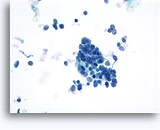
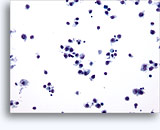
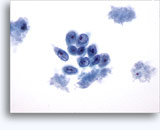
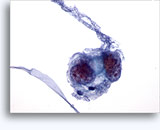
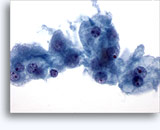
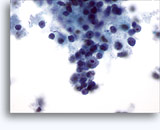
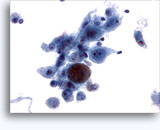
Figures 22-23: Small cells with hyperchromatic and smudgy molded nuclei, scant cytoplasm and high N/C ratios. Background contains necrotic granular debris.
Ovary and Testis FNAB
Ovarian and testicular FNAB are only rarely used for primary diagnosis of gonadal neoplasms. In North America, FNAB plays a role in the diagnosis of recurrent, or metastatic gonadal malignancies. For this latter use, FNAB diagnostic sensitivity is reported to vary from 65-95%, with higher specificity (92-100%). Reasons for false negative outcome may include sampling error due to radiation induced fibrosis, size of mass, or presence of necrosis; and interpretation error (undercall as benign).
Selected Cytology of Ovary FNAB
- Serous Adenocarcinoma
- Cellular samples
- Papillae, some branched
- Some single cells
- Malignant nuclear criteria, but may be absent if low grade
- Intracytoplasmic vacuoles
- Psammoma bodies naked, or in cell groups (non-specific finding)
- MIMICS: other carcinomas (primary & secondary) if low grade, indistingushable from serous borderline tumor
Selected Cytology of Testis FNAB
- Seminoma
- Dispersed large cells and few clusters
- Stripped nuclei, delicate cytoplasm
- Primitive cells: central nuclei, fine vesicular chromatin, prominent central nucleolus
- Background lymphocytes, epithelioid histiocytes
- Tigroid background (frothy, band-like, may be absent or minimal in thin layer preparations)
- PLAP positive; LCA, AFP, cytokeratin negative
- MIMICS: other germ cell tumors, malignant lymphoma
- Embryonal Carcinoma
- Necrosis present
- Papillary, gland-like, or in sheets
- Pleomorphic nuclei, coarse chromatin, several prominent nucleoli
- PLAP cytokeratin positive; AFP may be positive, LCA negative
- MIMICS: other germ cell tumors, malignant lymphoma, melanoma, high grade carcinoma
Bone and Soft Tissue FNAB
FNAB of bone and soft tissue lesions is usually employed to confirm metastatic disease, usually a carcinoma. At some centers, FNAB may be used for diagnosis of selected primary bone and soft tissue sarcomas. Diagnostic accuracy is highest for FNAB of metastatic neoplasms (range 92-100%).
Cytology of Bone & Soft Tissue Metastasis FNAB
- Metastatic carcinoma
- Necrosis in some
- Malignant nuclear features
- Clusters of cells and single cells (dyshesion)
- Neuroendocrine differentiation: high N/C ratios, smudgy hyperchromatic molded nuclei with scant cytoplasm
- Glandular differentiation: intracytoplasmic vacuoles, cell balls, papillae, glands
- Squamous differentiation: keratinization, hard refractile cytoplasm
- Urothelial differentiation: cercariform cells (cytoplasmic tails)
- Cytokeratin positive usually
- Metastatic malignant melanoma
- Dyshesive single cells
- Malignant nuclear features, eccentric nuclei
- Range of patterns: small, spindle or epithelioid cells
- Nuclear size variation
- Nuclear pseudoinclusions with bi-, and multinucleation
- Intracytoplasmic dusty brown melanin pigment
- S-100, HMB-45, Melan-A positive (not always)
- Malignant lymphoma
- Dyshesive single cells
- Open granular chromatin
- Nucleoli based on nuclear membrane in some subtypes
- Nuclear membrane protrusions and irregularity
- Scant cytoplasm in some subtypes (high N/C ratios)
- Lymphoglandular bodies in background
- LCA positive, B or T cell lineage
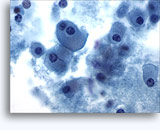
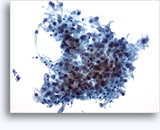
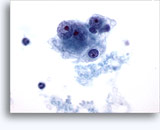
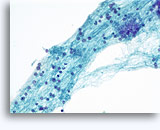
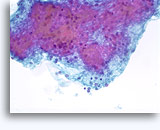
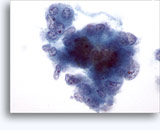
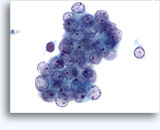
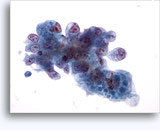
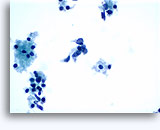
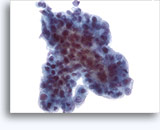
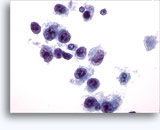
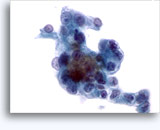
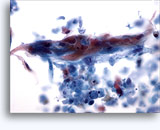
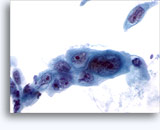
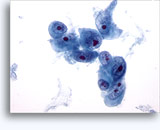
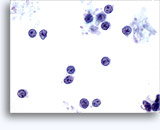
Figures 37-38: Dyshesive single cells with high N/C ratios, scant cytoplasm, open chromatin, membrane protrusions and membrane based nucleoli. Lymphoglandular bodies in background.
References
- Bommer KK, Ramzy I and Mody D. Fine-Needle Aspiration Biopsy in the Diagnosis and Management of Bone Lesions. Cancer (Cancer Cytopathology) 1997; 81:148-56.
- Caraway NP, Fanning CV, Amato RJ and Sneige N. Fine-Needle Aspiration Cytology of Seminoma: A Review of 16 Cases. Diagnostic Cytopathology 1995; 12:327-33.
- Geisinger KR et al. Modern Cytopathology. Philadelphia, Churchill Livingstone. 2004; pp 579-606, 689-700.
- Kabukcuoglu F, Kabukcuoglu Y, Kuzgun U and Evren I. Fine Needle aspiration of Malignant Bone Lesions. Acta Cytologica 1998;42:875-82.
- Liu J, Fanning CV. Can Renal Oncocytomas be Distinguished from Renal Cell Carcinoma on Fine-Needle Aspiration Specimens? Cancer (Cancer Cytopathol) 2001;93:390-7.
- Nguyen G and Akin MM. Fine Needle Aspiration Cytology of the Kidney, Renal Pelvis and Adrenal. In:Clinics in Laboratory Medicine. Stanley MW editor. Philadelphia. WB Saunders Company. 1998;18(3):429-60.
- Renshaw AA, Granter SR, Cibas ES. Fine-Needle Aspiration of the Adult Kidney. Cancer (Cancer Cytopathol) 1997;81:71-88.
- Renshaw, AA, Lee KR, Madge R, Granter SR. Accuracy of Fine Needle Aspiration in Distinguishing Subtypes of Renal Cell Carcinoma. Acta Cytol 1997;41:987-94.
- Wakely PE, Kneisl, JS. Soft Tissue Aspiration Cytopathology. Cancer (Cancer Cytopathol) 2000;90:292-8.
- Wiatrowska, BA, Zakowski MF. Fine-Needle Aspiration Biopsy of Chromophobe Renal Cell Carcinoma and Oncocytoma. Cancer (Cancer Cytopathol) 1999;87:161-7.
- Wu H H, Cramer HM, Kho J and Elsheikh, TM. Fine Needle Aspiration Cytology of Benign Adrenal Cortical Nodules. Acta Cytologica 1998;42:1352-8.
- Yang B, Syed ZA and Rosenthal DL. CD10 Facilitates the Diagnosis of Metastatic Renal Cell Carcinoma From Primary Adrenal Cortical Neoplasm in Adrenal Fine-Needle Aspiration. Diagn. Cytopathol. 2002;27:149-52.
- Zardauin IM. Renal FNAC Acta Cytol 1999;43:184-90.
- Zhang, PJ, Genega EM, Tomaszewski JE, Pasha TL, LiVolsi VA. The Role of Calretinin, Inhibin, Melan-A, BCL-2, and C-kit in Differentiating Adrenal Cortical and Medullary Tumors: An Immunohistochemical Study. Mod Pathol 2003;16(6):591-7.