Case Presentation
Case Presentation – June 2025
Nocardia
Written by: Kayleigh Bucci, Student, Cleveland Clinic School of Cytology, Cleveland, OH
Patient Age: 71-year-old male
Specimen Type: Right middle lobe of lung, FNA, Papanicolaou stained smears, ThinPrep® Non-Gyn slide, Cellient® cell block, concurrent surgical biopsy
Patient History: The patient has a history of nonischemic cardiomyopathy and is 8 months status post heart transplantation. He was admitted after finding a mass-like consolidation in the right lower lobe (RLL) on chest x-ray. Follow up chest CT showed mass-like consolidations measuring 4.9×7.7 cm in the right middle lobe (RML) and 3.6×3.4 cm in the RLL. These findings were concerning for post-transplant lymphoproliferative disorder (PTLD) versus infection.
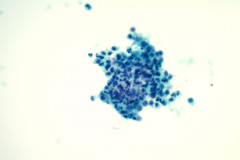
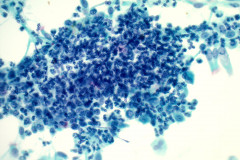
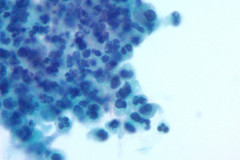
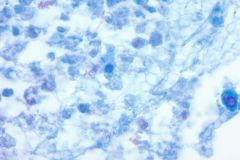
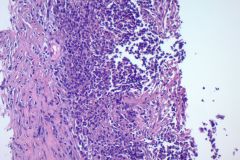
Cytologic Diagnosis: Negative for malignant cells, suppurative acute inflammation, reactive cellular changes. Filamentous bacteria present, consistent with Nocardia species.
Biopsy / Pathologic Diagnosis: Lung, RML, biopsy. Suppurative exudate with histiocytes. Filamentous bacteria present consistent with Nocardia. Bronchoscopy culture: 5,000 CFU/mL Nocardia nova
Case provided by: Cleveland Clinic, Cleveland, Ohio
Nocardia
Etiology:
Nocardia is an aerobic bacteria found in both fresh and saltwater, soil, dust, and decaying vegetation.1 The majority of infections are caused by inhalation of the bacteria, with the most common infection site being the lungs. The bacteria can also enter the body through lesions in the skin, causing cutaneous infection or possible central nervous system (CNS) infection.1 Immunocompromised individuals, frequently transplant or HIV positive patients, are most at risk for infection and disseminated disease.1
Clinical Features:
The clinical features of Nocardia infection are generalized and nonspecific, including fever, weight loss, night sweats, cough, and chest pain. Imaging may show irregular nodules, cavitary masses, diffuse pulmonary infiltrates, lung abscess, or pleural effusions.1 Nocardiosis is seen more frequently in males than females.2 In transplant patients, onset of infection has been seen 370 days (median) post-transplant. Diagnosis is often delayed, with symptoms occurring from one week to more than three months prior to diagnosis.2
Treatment and Prognosis:
Nocardiosis is most commonly treated with trimethoprim-sulfamethoxazole (TMP-SMX), although some species are resistant to this type of treatment.1 Some immunocompromised patients are placed on TMP-SMX prophylaxis to prevent infection, but studies have shown it is not always effective.2 Other forms of treatment include cotrimoxazole, imipenem, linezolid, and amikacin.3 The duration of treatment is 6-12 months for respiratory infections and a minimum of 12 months for CNS infections.1,2,3 Prognosis varies, with skin and soft tissue infections having an almost 100% cure rate, respiratory infections an 80% cure rate, and CNS infections 85% cured. The prognosis of disseminated infection is dismal with a mere 3% of patients cured.1
Cytology:
Nocardiosis causes suppurative inflammation with sheets of neutrophils in a necrotic background. Poorly formed granulomas may be seen. In some cases, the bacteria may be difficult to visualize due to obscuring inflammation.4 Papanicolaou stain will reveal thin, filamentous bacteria with a pale pink color.5 Nocardia organisms are 0.5-1.0 microns in diameter with 90-degree angle beaded branching.5 These filamentous bacteria stain positive on silver stain (GMS) which is very helpful for identifying the organisms. 5 They are partially acid fast and may stain positive on modified acid-fast stains such as a Fite stain. They are considered gram variable, some organisms staining gram positive and others gram negative in a tissue gram stain. A negative gram stain does not exclude the possibility of Nocardia. When possible Nocardia organisms are seen in cytology samples, the Microbiology lab should be notified to ensure appropriate cultures.
Differential Diagnosis:
As seen in this case, it is possible for Nocardia to present on imaging as a cavitary mass, making malignancy a top differential before specimen samples are even collected for laboratory testing. Non-malignant lesions such as bacterial, parasitic, and fungal infections can mimic malignant ones, particularly squamous cell carcinoma on CT and PET scans.6 After obtaining a specimen, making a specific diagnosis of Nocardia on morphology alone is nearly impossible; special stains must be used to differentiate Nocardia from other bacterial or fungal infections.
Actinomyces is a top differential of Nocardia as both are filamentous bacteria. Morphologically they are very similar, with Nocardia being 0.5-1.0 microns in diameter with 90-degree angle branching compared to 1.0-1.5 microns in diameter and acute angle branching for Actinomyces. On a Papanicolaou stain, Actinomyces should appear as a fuzzy purple aggregate with thin filamentous bacteria best seen at the periphery compared to the pale pink filamentous appearance of Nocardia.5 Both organisms stain positively with GMS, differentiating them from other bacterial species. Modified AFB (Fite) staining is used to differentiate between these two bacterial species. Nocardia is weakly positive for Fite staining whereas Actinomyces is negative.5 Ultimately, microbiology cultures are necessary to confirm the bacterial species is present and ensure the patient receives proper treatment.
Another potential differential is Aspergillus. The filamentous and branching nature of Nocardia may resemble fungal hyphae, but fungal infections will show true hyphae with septations and are much larger in diameter.5 Nocardia and Actinomyces are the only bacteria that are positive for GMS staining, typically GMS positivity is reserved for fungi. Positive gram staining is helpful in the ruling out fungal infections in these cases.5
Squamous cell carcinoma (SCC) is an important differential to consider, as treatment will vary drastically with a diagnosis of malignancy. SCC often presents as a cavitary lesion on imaging, and cytology will reveal a background of necrosis and neutrophils.7 The malignant cells will have dense cytoplasm with possible keratinization, angulated nuclear contours, coarse irregular chromatin, and bizarre cells shapes in a predominant single cell pattern with occasional sheets.7
References:
- Rathish B, Zito PM. Nocardia. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560872/
- Hemmersbach-Miller M, Stout JE, Woodworth MH, Cox GM, Saullo JL. Nocardia infections in the transplanted host. Transpl Infect Dis. 2018; 20:e12902. https://doi.org/10.1111/tid.12902
- Besteiro B, Coutinho D, Fragoso J, Figueiredo C, Nunes S, Azevedo C, Teixeira T, Selaru A, Abreu G, Malheiro L. Nocardiosis: a single-center experience and literature review. Braz J Infect Dis. 2023 Sep-Oct;27(5):102806. doi: 10.1016/j.bjid.2023.102806. Epub 2023 Oct 3. PMID: 37802128; PMCID: PMC10582834.
- Sathyanarayanan, S., Gochhait, D., Stephen, N., Amalnath, D., Sistla, S. and Siddaraju, N. (2022), Cytology of primary nocardiosis of the lymph node: A rare report. Cytopathology, 33: 415-417. https://doi.org/10.1111/cyt.13101
- McHugh KE, Sturgis CD, Procop GW, Rhoads DD. The cytopathology of Actinomyces, Nocardia, and their mimickers. Diagnostic Cytopathology. 2017; 45: 1105–1115. https://doi.org/10.1002/dc.23816
- Nguyen NC, Abhishek K, Nyon S, Farghaly HR, Osman MM, Reimers HJ. Are there radiographic, metabolic, and prognostic differences between cavitary and noncavitary nonsmall cell lung carcinoma? A retrospective fluorodeoxyglucose positron emission tomography/computed tomography study. Ann Thorac Med. 2016 Jan-Mar;11(1):49-54. doi: 10.4103/1817-1737.165296. PMID: 26933457; PMCID: PMC4748615.
- Berezowska, S., Maillard, M., Keyter, M. and Bisig, B. (2024), Pulmonary squamous cell carcinoma and lymphoepithelial carcinoma – morphology, molecular characteristics and differential diagnosis. Histopathology, 84: 32-49. https://doi.org/10.1111/his.15076