Regulations and Licensures
A legislatively established process of credentialing which grants a practitioner the legal right to practice through law. It restricts this right to only those persons who hold a license. Some will protect both the title and practice of the profession (practice acts and laws). Others will protect only the title granted to practitioners (title acts or laws).Click here to close this window.
Terminology used by state licensing boards for various professions that define the procedures, actions, and processes that are permitted for the licensed individual. The scope of practice is limited to that which the law allows for specific education and experience, and specific demonstrated competency. Each state has laws, licensing bodies, and regulations that describe requirements for education and training, and define scope of practice.Click here to close this window.
Click any blue labeled state code for regulations/licensure for that state. States without current regulations are not highlighted.
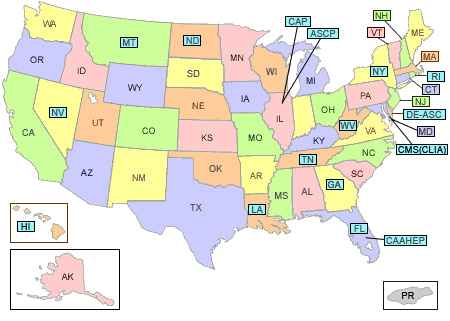
Introduction
At this time, California is the only state that does not allow HPV testing by cytotechnologists. Tennessee permits cytotechnologists to perform molecular testing if they have a special analyst license in molecular diagnostics (i.e., MP (ASCP) certification). From time to time, other states introduce licensure legislation. Illinois, Indiana, Massachusetts, Minnesota, Missouri, Pennsylvania, Iowa, and Vermont have introduced legislation or are considering doing so.
The information provided here is for reference and general knowledge purposes only. Please review the full set of regulations and/or check with your legal advisor to ensure compliance.
Relevant Definitions:
- Licensure: A legislatively established process of credentialing which grants a practitioner the legal right to practice through law. It restricts this right to only those persons who hold a license. Some will protect both the title and practice of the profession (practice acts and laws). Others will protect only the title granted to practitioners (title acts or laws).
- Scope of Practice: Terminology used by state licensing boards for various professions that define the procedures, actions, and processes that are permitted for the licensed individual. The scope of practice is limited to that which the law allows for specific education and experience, and specific demonstrated competency. Each state has laws, licensing bodies, and regulations that describe requirements for education and training, and define scope of practice.
