Fine needle aspiration (FNA) biopsies of the liver or pancreas are common procedures in many medical centers. The usual indication for a fine needle biopsy of either the liver or pancreas is evaluation of a mass lesion. In the past, pathologists have been more familiar with evaluating tissue core biopsies of liver masses, although FNA biopsy has become the standard procedure in the majority of hospitals. FNA of the pancreas, however, is more common than core biopsies due to the potential complication of pancreatitis following a core biopsy. One of the advantages of FNA biopsies of the liver or pancreas is that multiple aspirates can be performed during a single procedure, which can potentially allow a more complete sampling of the mass.
Occasionally, a dominant nodule in cirrhosis may be aspirated as part of an evaluation of a mass lesion [1, 2] . An important cytologic feature of a cirrhotic nodule is the variable, rather than uniform appearance of the liver cells on ThinPrep slides. Occasionally, atypical features including nuclear enlargement, binucleation and anisonucleosis may be seen, which are helpful in making a diagnosis of cirrhosis, since the polymorphic appearance of the liver cell population is a feature of benign, reactive hepatocytes versus the uniform atypia of hepatocellular carcinoma. Besides the variable cellularity of the liver cells in cirrhosis, thin trabeculae and cords measuring no greater than two cells thick are present and the cells have low nuclear-to-cytoplasmic ratios. Bile ducts may also be seen, along with occasional inflammatory cells and fibrous tissue.[1, 2]
ThinPrep prepared aspirates of hepatocellular carcinoma show cells with uniformly high nuclear-to-cytoplasmic ratios arranged in complex branching, anastomosing thick trabeculae, having sharply demarcated peripheral borders. The trabeculae contain greater than four cells in thickness, and there is peripheral endothelial encirclement, as well as so called “transgressing” endothelial cells present. Scattered single cells and small groups are often present, along with stripped atypical hepatocytic nuclei. Occasionally, bile pigment may be seen [1, 2, 6, 7, 8, 9, 10, 11, 12, 13] .
Occasional variants of hepatocellular carcinoma may be encountered, including fibrolamellar hepatocellular carcinoma, small cell and clear cell subtypes and mixed hepatocellular/cholangiocarcinomas. Cytologic features of these variants are well described in standard textbooks.[1, 2]
Aspirates of benign hepatocellular tumors such as adenoma and focal nodular hyperplasia consist of liver cells indistinct from hepatocytes of the unremarkable surrounding liver on ThinPrep slides.[1, 2] Fragments of fibrous tissue and bile ducts are present in focal nodular hyperplasia but are not appreciated in adenomas.
Due to the lack of atypical findings, a specific diagnosis of adenoma or focal nodular hyperplasia can’t be made from fine needle aspiration specimens. Therefore, it is critical to correlate the cytologic findings with the placement of the needle.
Lastly, liver aspirates of metastatic carcinoma are quite common, since the liver is the second most common abdominal site for metastatic malignancy, following lymph nodes. Metastatic carcinomas account for more than 90% of all malignant hepatic neoplasms. Adenocarcinomas from various sites are the main histologic type of metastatic carcinoma to involve the liver, with colorectal carcinoma having an especially high prevalence. Cytomorphologic features of metastatic adenocarcinoma are quite similar to the cytologic features seen in primary cholangiocarcinoma. FNA preparations of adenocarcinomas usually consist of cohesive aggregates of malignant cells in which gland formation may be seen. Cuboidal to columnar-shaped cells having delicate pale cytoplasm and eccentrically placed, round to irregular nuclei with prominent nucleoli are present. Colorectal carcinoma often has an associated “dirty necrosis” in the background, in contrast to the clean background of cholangiocarcinoma. In addition, cancers of other cell types can be encountered including metastatic small cell carcinoma, melanoma, malignant lymphoma and mesenchymal tumors.[1, 2]
Percutaneous, intraoperative and endoscopic ultrasound (EUS)-guided FNA biopsies are increasingly being utilized to evaluate cystic and solid masses of the pancreas[1, 2, 14, 15] . FNA has distinct advantages over large core biopsies and wedge resections of the pancreas, since these latter procedures can potentially lead to pancreatitis and/or peritonitis due to the spillage of exocrine enzymes and bile.[16] FNA biopsy of the pancreas can also be useful for the diagnosis of an inflammatory pancreatic lesion, obviating the need for a surgical procedure. Therefore, the cytopathologist needs to know the cytologic features and spectrum of changes seen in benign ductal and acinar pancreatic epithelium.[1, 2, 17] Characteristically, pancreatic ductal cells are arranged in flat honeycomb sheets with evenly spaced, round to oval nuclei and well-defined cell borders. Acinar cells tend to be arranged in small cohesive aggregates, consisting of cells with small uniform, basally placed nuclei having finely granular to clumpy chromatin with inconspicuous nucleoli surrounded by a moderate amount of granular cytoplasm. Islet cells are generally not appreciated in the aspirates.[1, 2, 14, 18]
Aspirates of pancreatic pseudocysts generally have scant cellularity with few or no epithelial cells, although acute and chronic inflammatory cells, histiocytes, granulation tissue and background debris with fragments of calcification can be present.[1, 2] Cytomorphologic features of acute pancreatitis include moderate to high cellularity consisting predominately of neutrophils in the smears along with a “dirty” background.[1, 2] Ductal and/or acinar cells may show reparative and/or inflammatory atypia, and fat necrosis may be appreciated. In contrast, aspirates of chronic pancreatitis generally have relatively low cellularity with only a few ductal cells that tend not to show the degree of atypical and/or reactive features seen in acute pancreatitis.[1, 2] Chronic inflammatory cells will be present.
It is critical to appreciate the cytomorphologic features of pancreatic adenocarcinoma, since this is a common malignancy, accounting for approximately 3% of all cancers and 5% of cancer mortality.[18, 19] Nearly ¾ of all pancreatic adenocarcinomas are of the ductal type. Cytomorphologic features of ductal adenocarcinomas in ThinPrep slides include high cellularity with atypical cells arranged singly and in groups and clusters.[1, 2] Within the cell groups, a syncytial arrangement of the cells is present along with loss of nuclear polarity. Both nuclear and cytoplasmic enlargement may be seen. The nuclei tend to be hyperchromatic with irregular chromatin and nuclear borders, along with occasional nuclear grooves. Considerable variation in nuclear size can be seen, as well as prominent nucleoli. The background usually contains a tumor diathesis.
Uncommon variants of pancreatic adenocarcinoma include anaplastic carcinoma (pleomorphic giant cell carcinoma), which has a markedly pleomorphic population, including giant and/or spindle shaped malignant cells and the very rare osteoclastic-like giant cell tumor of the pancreas, which consists of scattered multinucleated osteoclastic-like giant cells with centrally clustered nuclei and a moderate amount of surrounding cytoplasm, as well as similar appearing mononucleated cells.[1, 2]
Aspirates of islet cell neoplasms tend to be cellular, consisting of a monomorphic population of uniform small cells arranged predominantly in a discohesive pattern. The tumor cells are small with round to oval nuclei with evenly dispersed, finely to coarsely granular chromatin and inconspicuous nucleoli. The nuclei are eccentrically placed within these cells. In contrast, aspirates of the very uncommon acinar cell carcinoma have cells arranged in a lobular fashion and have hyperchromatic nuclei with clumpy chromatin and prominent nucleoli.[1, 2] Both islet and acinar cells have granular cytoplasm. Cytomorphologic features of papillary-cystic tumor include papillary structures having fibrovascular cores lined by one or more layers of epithelial cells with pale chromatin, delicate nuclear membranes and occasional longitudinal nuclear grooves.[1, 2] Mucinous cystic neoplasms, in contrast, have nuclei that vary from bland, atypical and/or frankly malignant.[1, 2] The cells can be singly arranged, in flat honeycomb groups or three-dimensional clusters associated with abundant intracellular and/or extracellular mucinous material. Other unusual lesions that have been described in the FNA literature include aspirates of serous cystadenoma and pancreatoblastoma. [1, 2, 20] Lastly, FNA of metastatic malignancies to the pancreas can be encountered. [21]
Reminder: You may click on any slide image
for an enlarged view.
Liver
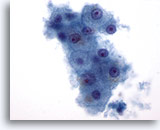
Figure 1: Liver FNA – Benign Hepatocytes. A small cluster of benign hepatocytes consisting of polygonal to round cells with well-defined cell borders and centrally placed nuclei with surrounding granular cytoplasm. There is also intracytoplasmic pigmentation present. Although small nucleoli are noted, there is no evidence of atypia or increased nuclear-to-cytoplasmic ratios. 60x
Figure 1
Liver FNA – Benign Hepatocytes
A small cluster of benign hepatocytes consisting of polygonal to round cells with well-defined cell borders and centrally placed nuclei with surrounding granular cytoplasm. There is also intracytoplasmic pigmentation present. Although small nucleoli are noted, there is no evidence of atypia or increased nuclear-to-cytoplasmic ratios.
60x
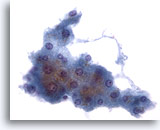
Figure 2: Liver FNA – Benign Hepatocytes. Cluster of benign reactive hepatocytes consisting of cuboidal shaped cells with well defined cell borders and vacuolated, to somewhat granular, opaque cytoplasm. Note the frayed edges of this group. The nuclei show some mild variation in size and an occasional binucleated cell is present. Small nucleoli are also noted. 40x
Figure 2
Liver FNA – Benign Hepatocytes
Cluster of benign reactive hepatocytes consisting of cuboidal shaped cells with well defined cell borders and vacuolated, to somewhat granular, opaque cytoplasm. Note the frayed edges of this group. The nuclei show some mild variation in size and an occasional binucleated cell is present. Small nucleoli are also noted.
40x
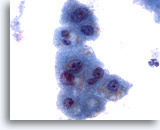
Figure 3
Liver FNA – Benign Hepatocytes
Note the slight variation in nuclear size, as well as the low nuclear-to-cytoplasmic ratios, which are typical of reactive hepatocytes. There is also evidence of fine cytoplasmic vacuolization and intracytoplasmic pigment. 60x
Figure 3
Liver FNA – Benign Hepatocytes
Note the slight variation in nuclear size, as well as the low nuclear-to-cytoplasmic ratios, which are typical of reactive hepatocytes. There is also evidence of fine cytoplasmic vacuolization and intracytoplasmic pigment.
60x
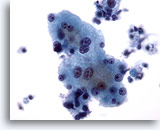
Figure 4
Liver FNA – Benign Hepatocytes
Reactive hepatocytes showing variation in nuclear size as well as binucleation. 40x
Figure 4
Liver FNA – Benign Hepatocytes
Reactive hepatocytes showing variation in nuclear size as well as binucleation.
40x
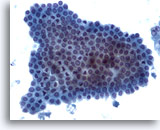
Figure 5
Liver FNA – Benign Ductal Cells
A cluster of benign bile duct cells presenting in a honeycomb arrangement with uniform small nuclei. 20x
Figure 5
Liver FNA – Benign Ductal Cells
A cluster of benign bile duct cells presenting in a honeycomb arrangement with uniform small nuclei.
20x
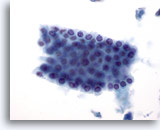
Figure 6
Liver FNA – Benign Ductal Cells. Group of benign bile ductal cells arranged in a honeycomb fashion. The cells are cuboidal with evenly spaced small nuclei. Ductal cells have higher nuclear-to-cytoplasmic ratios than hepatocytes and also lack cytoplasmic granularity, vacuolization and/or pigment. 40x
Figure 6
Liver FNA – Benign Ductal Cells
Group of benign bile ductal cells arranged in a honeycomb fashion. The cells are cuboidal with evenly spaced small nuclei. Ductal cells have higher nuclear-to-cytoplasmic ratios than hepatocytes and also lack cytoplasmic granularity, vacuolization and/or pigment.
40x
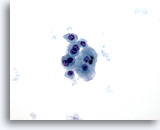
Figure 7
Liver FNA – Cirrhosis
Loose cluster of benign hepatocytes showing binucleation, with low nuclear-to-cytoplasmic ratios. 40x
Figure 7
Liver FNA – Cirrhosis
Loose cluster of benign hepatocytes showing binucleation, with low nuclear-to-cytoplasmic ratios.
40x
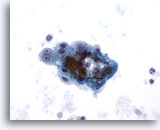
Figure 8
Liver FNA – Cirrhosis
Loose cluster of benign reactive hepatocytes from a cirrhotic nodule. Note the binucleation with prominent nucleoli, but the nuclear-to-cytoplasmic ratios remain low. 40x
Figure 8
Liver FNA – Cirrhosis
Loose cluster of benign reactive hepatocytes from a cirrhotic nodule. Note the binucleation with prominent nucleoli, but the nuclear-to-cytoplasmic ratios remain low.
40x
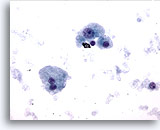
Figure 9
Liver FNA – Cirrhosis
Individually scattered, benign hepatocytes from a cirrhotic nodule. There is variation in nuclear size but no nuclear irregularity. A binucleated hepatocyte is present. The nuclear-to-cytoplasmic ratios remain low. 40x
Figure 9
Liver FNA – Cirrhosis
Individually scattered, benign hepatocytes from a cirrhotic nodule. There is variation in nuclear size but no nuclear irregularity. A binucleated hepatocyte is present. The nuclear-to-cytoplasmic ratios remain low.
40x
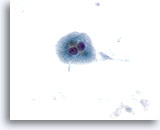
Figure 10
Liver FNA – Cirrhosis
Individually scattered benign binucleated hepatocytes from a cirrhotic nodule. 40x
Figure 10
Liver FNA – Cirrhosis
Individually scattered benign binucleated hepatocytes from a cirrhotic nodule.
40x
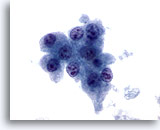
Figure 11
Liver FNA, Hepatocellular Carcinoma.
Hepatocytes from hepatocellular carcinoma demonstrating uniform atypicality, including increased nuclear-to-cytoplasmic ratios and nuclei possessing multiple irregular nucleoli. 60x
Figure 11
Liver FNA, Hepatocellular Carcinoma.
Hepatocytes from hepatocellular carcinoma demonstrating uniform atypicality, including increased nuclear-to-cytoplasmic ratios and nuclei possessing multiple irregular nucleoli.
60x
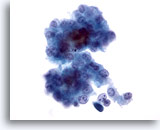
Figure 12
Liver FNA, Hepatocellular Carcinoma.
Loose cluster of malignant hepatocytes from an aspirate of hepatocellular carcinoma. There is uniform atypicality with increased nuclear-to-cytoplasmic ratios. Some bile pigment is noted between the hepatocytes. 40x
Figure 12
Liver FNA, Hepatocellular Carcinoma.
Loose cluster of malignant hepatocytes from an aspirate of hepatocellular carcinoma. There is uniform atypicality with increased nuclear-to-cytoplasmic ratios. Some bile pigment is noted between the hepatocytes.
40x
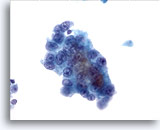
Figure 13
Liver FNA, Hepatocellular Carcinoma.
Well differentiated hepatocellular carcinoma in which the liver cells show increased nuclear-to-cytoplasmic ratios and uniform atypicality. 40x
Figure 13
Liver FNA, Hepatocellular Carcinoma.
Well differentiated hepatocellular carcinoma in which the liver cells show increased nuclear-to-cytoplasmic ratios and uniform atypicality.
40x
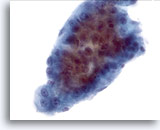
Figure 14
Liver FNA, Hepatocellular Carcinoma.
A cluster of hepatocellular carcinoma consisting of thick trabeculae with sharply demarcated peripheral borders typical of hepatocellular carcinoma. Uniform atypicality of the hepatocytes is present. 40x
Figure 14
Liver FNA, Hepatocellular Carcinoma.
A cluster of hepatocellular carcinoma consisting of thick trabeculae with sharply demarcated peripheral borders typical of hepatocellular carcinoma. Uniform atypicality of the hepatocytes is present.
40x
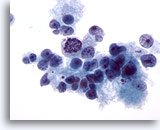
Figure 15
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma in this demonstrating loosely cohesive malignant hepatocytes, as well as considerable variation in nuclear size and shape. 40x
Figure 15
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma in this demonstrating loosely cohesive malignant hepatocytes, as well as considerable variation in nuclear size and shape.
40x
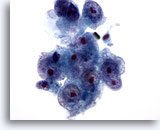
Figure 16
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma demonstrating hepatocytes with high nuclear to cytoplasmic ratios and nuclear irregularity. Multiple prominent nucleoli are present. 60x
Figure 16
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma demonstrating hepatocytes with high nuclear to cytoplasmic ratios and nuclear irregularity. Multiple prominent nucleoli are present.
60x
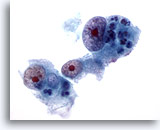
Figure 17
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma in which the hepatocytes show marked nuclear enlargement with nuclear irregularity and very prominent nucleoli. 60x
Figure 17
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma in which the hepatocytes show marked nuclear enlargement with nuclear irregularity and very prominent nucleoli.
60x
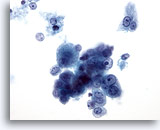
Figure 18: Liver FNA, Hepatocellular Carcinoma. Loose cluster of individually scattered malignant hepatocytes demonstrating uniform atypicality. The liver cells retain their hepatocytic features characterized by more cuboidal shape with centrally placed nucleoli. Binucleated tumor cells are noted. Increased nuclear-to-cytoplasmic ratios are present. 40x
Figure 18
Liver FNA, Hepatocellular Carcinoma.
Loose cluster of individually scattered malignant hepatocytes demonstrating uniform atypicality. The liver cells retain their hepatocytic features characterized by more cuboidal shape with centrally placed nucleoli. Binucleated tumor cells are noted. Increased nuclear-to-cytoplasmic ratios are present.
40x
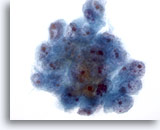
Figure 19
Liver FNA, Hepatocellular Carcinoma.
Cluster of malignant cells in aspirate of hepatocellular carcinoma. Note the nuclear enlargement, irregular chromatin distribution and the presence of one or more nucleoli, as well as increased nuclear-to-cytoplasmic ratios. 40x
Figure 19
Liver FNA, Hepatocellular Carcinoma.
Cluster of malignant cells in aspirate of hepatocellular carcinoma. Note the nuclear enlargement, irregular chromatin distribution and the presence of one or more nucleoli, as well as increased nuclear-to-cytoplasmic ratios.
40x
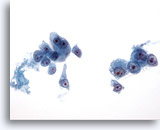
Figure 20
Liver FNA, Hepatocellular Carcinoma.
Loosely cohesive to single malignant cells in an aspirate of hepatocellular carcinoma. There is uniform atypicality in which the malignant cells have increased nuclear-to-cytoplasmic ratios. Note the prominent nucleoli present. 40x
Figure 20
Liver FNA, Hepatocellular Carcinoma.
Loosely cohesive to single malignant cells in an aspirate of hepatocellular carcinoma. There is uniform atypicality in which the malignant cells have increased nuclear-to-cytoplasmic ratios. Note the prominent nucleoli present.
40x
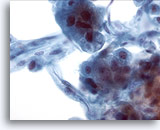
Figure 21
Liver FNA, Hepatocellular Carcinoma.
Aspirate of hepatocellular carcinoma in which hepatocytes and peripherally located endothelial cells are noted. Endothelial cells are characterized by spindle shaped cells having elongated nuclei and indistinct cytoplasm. 40x
Figure 21
Liver FNA, Hepatocellular Carcinoma.
Aspirate of hepatocellular carcinoma in which hepatocytes and peripherally located endothelial cells are noted. Endothelial cells are characterized by spindle shaped cells having elongated nuclei and indistinct cytoplasm.
40x
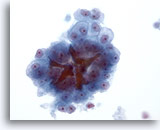
Figure 22
Liver FNA, Hepatocellular Carcinoma.
Hepatocellular carcinoma consisting of atypical hepatocytes demonstrating uniform atypicality as well as bile plugs. 40x
Figure 22
Liver FNA, Hepatocellular Carcinoma.
Hepatocellular carcinoma consisting of atypical hepatocytes demonstrating uniform atypicality as well as bile plugs.
40x
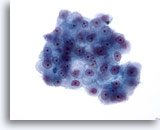
Figure 23
Liver FNA, Hepatocellular Carcinoma. Cluster of malignant cells having increased nuclear-to-cytoplasmic ratios with nuclei varying from oval to somewhat irregular. Prominent nucleoli are also present. The cytoplasm varies from granular to pale, and sharp cytoplasmic borders are present. 40x
Figure 23
Liver FNA, Hepatocellular Carcinoma.
Cluster of malignant cells having increased nuclear-to-cytoplasmic ratios with nuclei varying from oval to somewhat irregular. Prominent nucleoli are also present. The cytoplasm varies from granular to pale, and sharp cytoplasmic borders are present.
40x
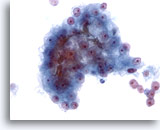
Figure 24
Liver FNA, Hepatocellular Carcinoma.
Cluster of malignant cells in an aspirate of hepatocellular carcinoma in which bile plugging is seen. Although the presence of bile is a specific feature of hepatocellular carcinoma, it is seen in only a minority of cases. 40x
Figure 24
Liver FNA, Hepatocellular Carcinoma.
Cluster of malignant cells in an aspirate of hepatocellular carcinoma in which bile plugging is seen. Although the presence of bile is a specific feature of hepatocellular carcinoma, it is seen in only a minority of cases.
40x
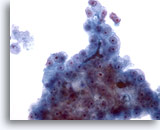
Figure 25
Liver FNA, Hepatocellular Carcinoma.
Another example of bile plugging in an aspirate of hepatocellular carcinoma. 40x
Figure 25
Liver FNA, Hepatocellular Carcinoma.
Another example of bile plugging in an aspirate of hepatocellular carcinoma.
40x
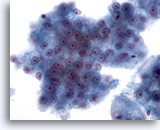
Figure 26
Liver FNA, Hepatocellular Carcinoma.
Clusters of malignant hepatocytes showing uniform atypicality. 40x
Figure 26
Liver FNA, Hepatocellular Carcinoma.
Clusters of malignant hepatocytes showing uniform atypicality.
40x
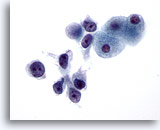
Figure 27
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma having a dissociative pattern with malignant cells showing markedly increased nuclear to cytoplasmic ratios. 60x
Figure 27
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma having a dissociative pattern with malignant cells showing markedly increased nuclear to cytoplasmic ratios.
60x
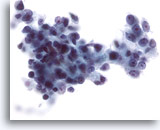
Figure 28
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma in which the malignant cells have markedly increased nuclear-to-cytoplasmic ratios. Considerable nuclear irregularity is also noted. 40x
Figure 28
Liver FNA, Hepatocellular Carcinoma.
Poorly differentiated hepatocellular carcinoma in which the malignant cells have markedly increased nuclear-to-cytoplasmic ratios. Considerable nuclear irregularity is also noted.
40x
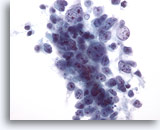
Figure 29: Liver FNA, Hepatocellular Carcinoma. Aspirate of hepatocellular carcinoma in which the tumor cells are loosely cohesive. As hepatocellular carcinomas get more poorly differentiated, they show a greater degree of discohesion, and lose some of the “hepatocellular” features. Therefore, the differential diagnosis of a primary hepatocellular carcinoma versus a metastatic carcinoma can often be challenging. 40x
Figure 29
Liver FNA, Hepatocellular Carcinoma.
Aspirate of hepatocellular carcinoma in which the tumor cells are loosely cohesive. As hepatocellular carcinomas get more poorly differentiated, they show a greater degree of discohesion, and lose some of the “hepatocellular” features. Therefore, the differential diagnosis of a primary hepatocellular carcinoma versus a metastatic carcinoma can often be challenging.
40x
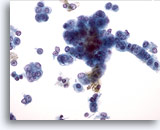
Figure 30
Liver FNA, Hepatocellular Carcinoma. Malignant hepatocytes in which there is increased nuclear-to-cytoplasmic ratios and nuclei with a vesicular chromatic pattern and prominent nucleoli. The linear arrangement of the tumor cells on the right, as well as their more columnar shape raises the possibility of a mixed hepatocellular/ cholangiocarcinoma. 40x
Figure 30
Liver FNA, Hepatocellular Carcinoma.
Malignant hepatocytes in which there is increased nuclear-to-cytoplasmic ratios and nuclei with a vesicular chromatic pattern and prominent nucleoli. The linear arrangement of the tumor cells on the right, as well as their more columnar shape raises the possibility of a mixed hepatocellular/ cholangiocarcinoma.
40x
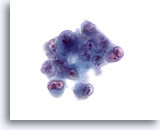
Figure 31
Liver FNA, Hepatocellular Carcinoma.
Aspirate of poorly differentiated hepatocellular carcinoma in which there are very atypical cells showing high nuclear-to-cytoplasmic ratios, bi- to multinucleation and irregular chromatin clumping with prominent irregular nucleoli. 60x
Figure 31
Liver FNA, Hepatocellular Carcinoma.
Aspirate of poorly differentiated hepatocellular carcinoma in which there are very atypical cells showing high nuclear-to-cytoplasmic ratios, bi- to multinucleation and irregular chromatin clumping with prominent irregular nucleoli.
60x
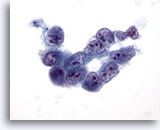
Figure 32
Liver FNA, Hepatocellular Carcinoma. Aspirate of poorly differentiated hepatocellular carcinoma in which a loosely cohesive group of malignant cells show very high nuclear-to-cytoplasmic ratios, nuclear irregularity and irregular chromatin distribution with multiple nucleoli. 60x
Figure 32
Liver FNA, Hepatocellular Carcinoma.
Aspirate of poorly differentiated hepatocellular carcinoma in which a loosely cohesive group of malignant cells show very high nuclear-to-cytoplasmic ratios, nuclear irregularity and irregular chromatin distribution with multiple nucleoli.
60x
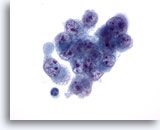
Figure 33
Liver FNA, Hepatocellular Carcinoma.
Aspirate of poorly differentiated hepatocellular carcinoma demonstrating malignant cells showing variation in size and shape with nuclei that also are irregular with irregular chromatin distribution and multiple nucleoli. 60x
Figure 33
Liver FNA, Hepatocellular Carcinoma.
Aspirate of poorly differentiated hepatocellular carcinoma demonstrating malignant cells showing variation in size and shape with nuclei that also are irregular with irregular chromatin distribution and multiple nucleoli.
60x
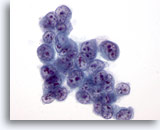
Figure 34
Liver FNA, Hepatocellular Carcinoma.
Aspirate of hepatocellular carcinoma in which the tumor cells have very high nuclear-to-cytoplasmic ratios. 60x
Figure 34
Liver FNA, Hepatocellular Carcinoma.
Aspirate of hepatocellular carcinoma in which the tumor cells have very high nuclear-to-cytoplasmic ratios.
60x
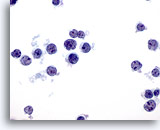
Figure 35
Liver FNA – Lymphoma
In this slide atypical lymphoid cells are present, arranged in a dissociative fashion. Note the high nuclear to cytoplasmic ratios with nuclear irregularity and small but prominent nucleoli. 60x
Figure 35
Liver FNA – Lymphoma
In this slide atypical lymphoid cells are present, arranged in a dissociative fashion. Note the high nuclear to cytoplasmic ratios with nuclear irregularity and small but prominent nucleoli.
60x
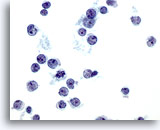
Figure 36
Liver FNA – Lymphoma. Aspirate of non-Hodgkin’s lymphoma in which the tumor cells are individually scattered and show high nuclear-to-cytoplasmic ratios with nuclear irregularity including clefts and snouts. Some of the tumor cells have small, but prominent nucleoli. 60x
Figure 36
Liver FNA – Lymphoma
Aspirate of non-Hodgkin’s lymphoma in which the tumor cells are individually scattered and show high nuclear-to-cytoplasmic ratios with nuclear irregularity including clefts and snouts. Some of the tumor cells have small, but prominent nucleoli.
60x
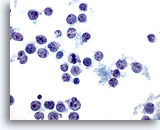
Figure 37
Liver FNA – Lymphoma
Aspirate of non-Hodgkin’s lymphoma in which individually scattered malignant lymphoid cells are present demonstrating oval to irregular nuclei with nuclear clefts and snouts, and irregular nuclear membranes. 60x
Figure 37
Liver FNA – Lymphoma
Aspirate of non-Hodgkin’s lymphoma in which individually scattered malignant lymphoid cells are present demonstrating oval to irregular nuclei with nuclear clefts and snouts, and irregular nuclear membranes.
60x
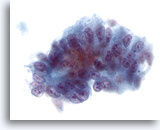
Figure 38
Liver FNA, Metastatic Adenocarcinoma.
Metastatic adenocarcinoma to the liver consisting of atypical columnar shaped cells with suggestive pseudostratification of the nuclei. The nuclei are oval with multiple nucleoli and nuclear irregularity. 40x
Figure 38
Liver FNA, Metastatic Adenocarcinoma.
Metastatic adenocarcinoma to the liver consisting of atypical columnar shaped cells with suggestive pseudostratification of the nuclei. The nuclei are oval with multiple nucleoli and nuclear irregularity.
40x
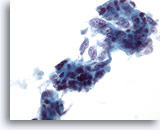
Figure 39
Liver FNA, Metastatic Adenocarcinoma.
Loose clusters of metastatic adenocarcinoma in which many of the tumor cells have a columnar shape with elongated vesicular nuclei and multiple prominent nucleoli. 40x
Figure 39
Liver FNA, Metastatic Adenocarcinoma.
Loose clusters of metastatic adenocarcinoma in which many of the tumor cells have a columnar shape with elongated vesicular nuclei and multiple prominent nucleoli.
40x
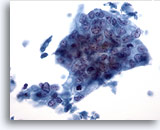
Figure 40
Liver FNA, Metastatic Adenocarcinoma.
Poorly differentiated adenocarcinoma in which the tumor cells are arranged in a syncytial fashion with the nuclei demonstrating loss of polarity. The tumor cells show considerable nuclear variation in size and shape. 40x
Figure 40
Liver FNA, Metastatic Adenocarcinoma.
Poorly differentiated adenocarcinoma in which the tumor cells are arranged in a syncytial fashion with the nuclei demonstrating loss of polarity. The tumor cells show considerable nuclear variation in size and shape.
40x
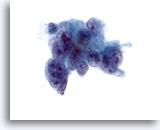
Figure 41
Liver FNA, Metastatic Adenocarcinoma. Aspirate of metastatic adenocarcinoma in which poorly differentiated malignant cells are noted showing considerable variation in nuclear size and shape. Multinucleation is also noted. Irregular chromatin distribution is seen with one or more prominent nucleoli. The tumor cells have delicate cytoplasm. 60x
Figure 41
Liver FNA, Metastatic Adenocarcinoma.
Aspirate of metastatic adenocarcinoma in which poorly differentiated malignant cells are noted showing considerable variation in nuclear size and shape. Multinucleation is also noted. Irregular chromatin distribution is seen with one or more prominent nucleoli. The tumor cells have delicate cytoplasm.
60x
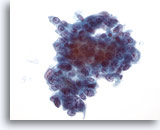
Figure 42
Liver FNA, Metastatic Adenocarcinoma.
Aspirate of metastatic adenocarcinoma in which the tumor cells vary from cuboidal to somewhat columnar. The nuclei show some variation in size and shape and have a vesicular chromatin distribution with prominent nucleoli. 40x
Figure 42
Liver FNA, Metastatic Adenocarcinoma.
Aspirate of metastatic adenocarcinoma in which the tumor cells vary from cuboidal to somewhat columnar. The nuclei show some variation in size and shape and have a vesicular chromatin distribution with prominent nucleoli.
40x
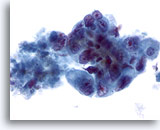
Figure 43
Liver FNA, Metastatic Adenocarcinoma.
Poorly differentiated malignant cells showing considerable variation in nuclear size and shape, along with nuclei having irregular chromatin distribution and prominent nucleoli. 40x
Figure 43
Liver FNA, Metastatic Adenocarcinoma.
Poorly differentiated malignant cells showing considerable variation in nuclear size and shape, along with nuclei having irregular chromatin distribution and prominent nucleoli.
40x
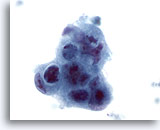
Figure 44
Liver FNA, Metastatic Adenocarcinoma.
Poorly differentiated malignant cells in which a syncytial arrangement is noted, as well as a loss of polarity. Marked nuclear irregularity, hyperchromasia and irregular nucleoli are present. 60x
Figure 44
Liver FNA, Metastatic Adenocarcinoma.
Poorly differentiated malignant cells in which a syncytial arrangement is noted, as well as a loss of polarity. Marked nuclear irregularity, hyperchromasia and irregular nucleoli are present.
60x
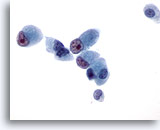
Figure 45
Liver FNA, Metastatic Cholangiocarcinoma.
The malignant cells have cuboidal to columnar shapes with eccentrically placed nuclei possessing prominent irregular nucleoli. 60x
Figure 45
Liver FNA, Metastatic Cholangiocarcinoma.
The malignant cells have cuboidal to columnar shapes with eccentrically placed nuclei possessing prominent irregular nucleoli.
60x
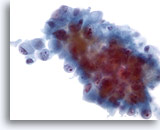
Figure 46
Liver FNA, Metastatic Cholangiocarcinoma. Aspirate of metastatic cholangiocarcinoma in which the tumor cells vary from oval to columnar. The tumor cells have nuclei that are eccentrically placed. Nuclear irregularity, irregular chromatin distribution and prominent nucleoli are noted. Surrounding delicate amphophilic cytoplasm is present. 40x
Figure 46
Liver FNA, Metastatic Cholangiocarcinoma.
Aspirate of metastatic cholangiocarcinoma in which the tumor cells vary from oval to columnar. The tumor cells have nuclei that are eccentrically placed. Nuclear irregularity, irregular chromatin distribution and prominent nucleoli are noted. Surrounding delicate amphophilic cytoplasm is present.
40x
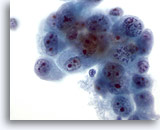
Figure 47
Liver FNA, Metastatic Cholangiocarcinoma. Aspirate of metastatic cholangiocarcinoma with oval to irregularly shaped malignant cells demonstrating clumpy chromatin which is irregularly distributed. The tumor cells possess multiple nucleoli. Surrounding amphophilic delicate cytoplasm is present. 60x
Figure 47
Liver FNA, Metastatic Cholangiocarcinoma.
Aspirate of metastatic cholangiocarcinoma with oval to irregularly shaped malignant cells demonstrating clumpy chromatin which is irregularly distributed. The tumor cells possess multiple nucleoli. Surrounding amphophilic delicate cytoplasm is present.
60x
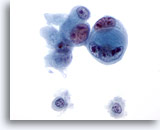
Figure 48
Liver FNA, Metastatic Cholangiocarcinoma.
Poorly differentiated adenocarcinoma in which a cell-within-cell arrangement is noted. Considerable variation in nuclear size and shape is present as well as multiple, irregular nucleoli. 60x
Figure 48
Liver FNA, Metastatic Cholangiocarcinoma.
Poorly differentiated adenocarcinoma in which a cell-within-cell arrangement is noted. Considerable variation in nuclear size and shape is present as well as multiple, irregular nucleoli.
60x
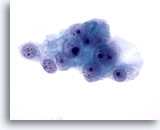
Figure 49
Liver FNA, Metastatic Renal Cell Carcinoma.
The malignant cells have round nuclei with multiple nucleoli and surrounding pale delicate cytoplasm with frayed borders. 60x
Figure 49
Liver FNA, Metastatic Renal Cell Carcinoma.
The malignant cells have round nuclei with multiple nucleoli and surrounding pale delicate cytoplasm with frayed borders.
60x
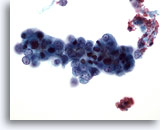
Figure 50
Liver FNA, Metastatic Endometrial Adenocarcinoma.
Aspirate of metastatic endometrial carcinoma in which relatively small malignant cells are present having very high nuclear to cytoplasmic ratios. 40x
Figure 50
Liver FNA, Metastatic Endometrial Adenocarcinoma.
Aspirate of metastatic endometrial carcinoma in which relatively small malignant cells are present having very high nuclear to cytoplasmic ratios.
40x
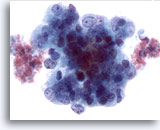
Figure 51
Liver FNA, Metastatic Endometrial Adenocarcinoma.
Aspirate of metastatic endometrial adenocarcinoma to the liver in which the tumor cells demonstrate marked variation in nuclear size and shape. The tumor cells have irregular chromatin and prominent nucleoli. 60x
Figure 51
Liver FNA, Metastatic Endometrial Adenocarcinoma.
Aspirate of metastatic endometrial adenocarcinoma to the liver in which the tumor cells demonstrate marked variation in nuclear size and shape. The tumor cells have irregular chromatin and prominent nucleoli.
60x
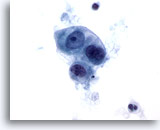
Figure 52
Metastatic Cecal Carcinoma
Metastatic adenocarcinoma of the cecum in which a loose cluster of cuboidal to oval malignant cells are present. There is variation in nuclear size and shape. 60x
Figure 52
Metastatic Cecal Carcinoma
Metastatic adenocarcinoma of the cecum in which a loose cluster of cuboidal to oval malignant cells are present. There is variation in nuclear size and shape.
60x
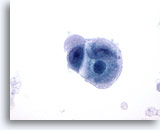
Figure 53
Metastatic Cecal Carcinoma
Fine needle aspiration of a metastatic poorly differentiated carcinoma of the cecum in which the tumor cells show variation in nuclear size and shape. The surrounding cytoplasm has a bubbly amphophilic appearance. 60x
Figure 53
Metastatic Cecal Carcinoma
Fine needle aspiration of a metastatic poorly differentiated carcinoma of the cecum in which the tumor cells show variation in nuclear size and shape. The surrounding cytoplasm has a bubbly amphophilic appearance.
60x
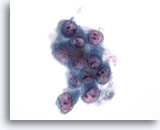
Figure 54: Liver FNA, Metastatic Lung Carcinoma. Fine needle aspiration of a metastatic adenocarcinoma of the lung to the liver in which a loose cluster of malignant cells shows considerable variation in nuclear shape. High nuclear-to-cytoplasmic ratios are noted. The individual tumor cells have an eccentrically placed nucleus with surrounding amphophilic cytoplasm. 60x
Figure 54
Liver FNA, Metastatic Lung Carcinoma.
Fine needle aspiration of a metastatic adenocarcinoma of the lung to the liver in which a loose cluster of malignant cells shows considerable variation in nuclear shape. High nuclear-to-cytoplasmic ratios are noted. The individual tumor cells have an eccentrically placed nucleus with surrounding amphophilic cytoplasm.
60x
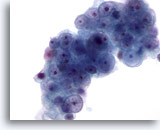
Figure 55
Liver FNA, Metastatic Renal Cell Carcinoma to the Liver. Metastatic renal cell carcinoma to the liver in which the tumor cells have round nuclei that vary in size with little nuclear membrane irregularity. The nuclei possess small to prominent nucleoli. Surrounding pale cytoplasm is present. 60x
Figure 55
Liver FNA, Metastatic Renal Cell Carcinoma to the Liver.
Metastatic renal cell carcinoma to the liver in which the tumor cells have round nuclei that vary in size with little nuclear membrane irregularity. The nuclei possess small to prominent nucleoli. Surrounding pale cytoplasm is present.
60x
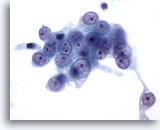
Figure 56
Liver FNA, Metastatic Renal Cell Carcinoma to the Liver. Fine needle aspiration of a metastatic renal cell carcinoma in which the malignant cells have round nuclei with a vesicular chromatin pattern and one or more prominent nucleoli. Note the surrounding pale cytoplasm and frayed cytoplasmic borders. 60x
Figure 56
Liver FNA, Metastatic Renal Cell Carcinoma to the Liver.
Fine needle aspiration of a metastatic renal cell carcinoma in which the malignant cells have round nuclei with a vesicular chromatin pattern and one or more prominent nucleoli. Note the surrounding pale cytoplasm and frayed cytoplasmic borders.
60x
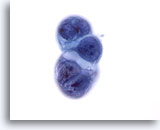
Figure 57
Liver FNA, Metastatic Pancreatic Carcinoma.
A cluster of ductal carcinoma of the pancreas metastatic to the liver. High nuclear-to-cytoplasmic ratios, as well as nuclear irregularity and clumpy chromatin are present. Cytoplasmic vacuolization is also noted. 60x
Figure 57
Liver FNA, Metastatic Pancreatic Carcinoma.
A cluster of ductal carcinoma of the pancreas metastatic to the liver. High nuclear-to-cytoplasmic ratios, as well as nuclear irregularity and clumpy chromatin are present. Cytoplasmic vacuolization is also noted.
60x
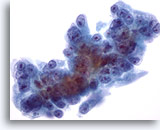
Figure 58
Liver FNA, Metastatic Pancreatic Carcinoma.
Metastatic pancreatic carcinoma to the liver in which the malignant cells show considerable variation in nuclear size and shape. Eccentrically placed nucleoli are seen surrounded by amphophilic, delicate cytoplasm. 60x
Figure 58
Liver FNA, Metastatic Pancreatic Carcinoma.
Metastatic pancreatic carcinoma to the liver in which the malignant cells show considerable variation in nuclear size and shape. Eccentrically placed nucleoli are seen surrounded by amphophilic, delicate cytoplasm.
60x
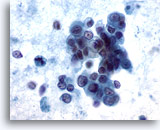
Figure 59
Liver FNA, Metastatic Pancreatic Carcinoma. Loose clusters of malignant cells having pale to vacuolated cytoplasm and eccentrically placed nuclei. Considerable variation in nuclear size and shape is seen. Besides loosely cohesive groups, some individual tumor cells are noted. Considerable tumor diathesis is noted in the background. 60x
Figure 59
Liver FNA, Metastatic Pancreatic Carcinoma.
Loose clusters of malignant cells having pale to vacuolated cytoplasm and eccentrically placed nuclei. Considerable variation in nuclear size and shape is seen. Besides loosely cohesive groups, some individual tumor cells are noted. Considerable tumor diathesis is noted in the background.
60x
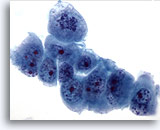
Figure 60
Liver FNA, Metastatic Pancreatic Carcinoma.
Poorly differentiated malignant cells showing considerable variation in nuclear size and shape. Irregular chromatin distribution and prominent nucleoli are noted. 60x
Figure 60
Liver FNA, Metastatic Pancreatic Carcinoma.
Poorly differentiated malignant cells showing considerable variation in nuclear size and shape. Irregular chromatin distribution and prominent nucleoli are noted.
60x
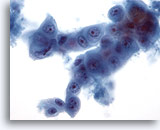
Figure 61
Liver FNA, Metastatic Pancreatic Carcinoma.
Aspirate of metastatic pancreatic carcinoma in which oval to cuboidal cells are present including the presence of a cell-within-a-cell arrangement. Considerable variation in nuclear size and shape is present. 40x
Figure 61
Liver FNA, Metastatic Pancreatic Carcinoma.
Aspirate of metastatic pancreatic carcinoma in which oval to cuboidal cells are present including the presence of a cell-within-a-cell arrangement. Considerable variation in nuclear size and shape is present.
40x
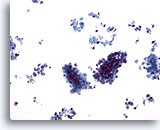
Figure 62
Liver FNA, Metastatic Small Cell Carcinoma.
Clusters of malignant small cells, as well as individually scattered tumor cells are present. Note the characteristic small size of the malignant cells, as well as the high nuclear to cytoplasmic ratios. 20x
Figure 62
Liver FNA, Metastatic Small Cell Carcinoma.
Clusters of malignant small cells, as well as individually scattered tumor cells are present. Note the characteristic small size of the malignant cells, as well as the high nuclear to cytoplasmic ratios.
20x
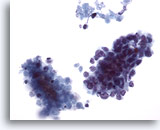
Figure 63
Liver FNA, Metastatic Small Cell Carcinoma. Clusters of metastatic small cell carcinoma of the lung in which small malignant cells have high nuclear to cytoplasmic ratios. Hyperchromatic nuclei lacking prominent nucleoli are present. Note the necrotic material adjacent to the malignant cluster. 40x
Figure 63
Liver FNA, Metastatic Small Cell Carcinoma.
Clusters of metastatic small cell carcinoma of the lung in which small malignant cells have high nuclear to cytoplasmic ratios. Hyperchromatic nuclei lacking prominent nucleoli are present. Note the necrotic material adjacent to the malignant cluster.
40x
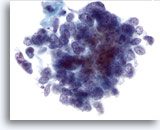
Figure 64
Liver FNA, Metastatic Small Cell Carcinoma.
Note the evenly distributed chromatin and lack of prominent nucleoli typical of small cell carcinoma, along with the high nuclear to cytoplasmic ratios. 60x
Figure 64
Liver FNA, Metastatic Small Cell Carcinoma.
Note the evenly distributed chromatin and lack of prominent nucleoli typical of small cell carcinoma, along with the high nuclear to cytoplasmic ratios.
60x
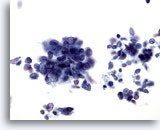
Figure 65
Liver FNA, Metastatic Small Cell Carcinoma. Aspirate of metastatic small cell carcinoma of the lung in which loose clusters of small malignant cells are present having high nuclear to cytoplasmic ratios. For the most part, nucleoli are not evident. A finely distributed chromatin pattern is noted. 40x
Figure 65
Liver FNA, Metastatic Small Cell Carcinoma.
Aspirate of metastatic small cell carcinoma of the lung in which loose clusters of small malignant cells are present having high nuclear to cytoplasmic ratios. For the most part, nucleoli are not evident. A finely distributed chromatin pattern is noted.
40x
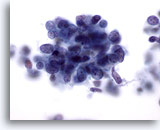
Figure 66
Liver FNA, Metastatic Small Cell Carcinoma.
High power of a metastatic small cell carcinoma of the lung in which malignant cells have high nuclear-to-cytoplasmic ratios with nuclei having finely granular, evenly distributed chromatin lacking prominent nucleoli. 60x
Figure 66
Liver FNA, Metastatic Small Cell Carcinoma.
High power of a metastatic small cell carcinoma of the lung in which malignant cells have high nuclear-to-cytoplasmic ratios with nuclei having finely granular, evenly distributed chromatin lacking prominent nucleoli.
60x
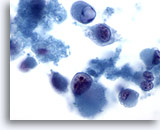
Figure 67
Liver FNA, Metastatic Melanoma
Dissociative smear in which markedly atypical cells having a rhabdoid phenotype are seen. Rhabdoid phenotype is characterized by large intracytoplasmic globules, as well as nuclei possessing prominent nucleoli. 60x
Figure 67
Liver FNA, Metastatic Melanoma
Dissociative smear in which markedly atypical cells having a rhabdoid phenotype are seen. Rhabdoid phenotype is characterized by large intracytoplasmic globules, as well as nuclei possessing prominent nucleoli.
60x
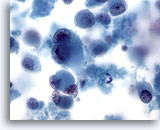
Figure 68
Liver FNA, Metastatic Melanoma.
Aspirate of metastatic melanoma to the liver in which individually scattered malignant cells are present, including some demonstrating characteristic binucleation with prominent nucleoli. As seen in some metastatic melanomas, a moderate amount of cytoplasm is present. 60x
Figure 68
Liver FNA, Metastatic Melanoma
Aspirate of metastatic melanoma to the liver in which individually scattered malignant cells are present, including some demonstrating characteristic binucleation with prominent nucleoli. As seen in some metastatic melanomas, a moderate amount of cytoplasm is present.
60x
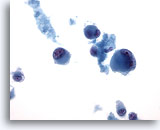
Figure 69
Liver FNA, Metastatic Melanoma
Aspirate of metastatic malignant melanoma to the liver in which individually scattered bizarre tumor cells are present, including some demonstrating binucleation. 40x
Figure 69
Liver FNA, Metastatic Melanoma
Aspirate of metastatic malignant melanoma to the liver in which individually scattered bizarre tumor cells are present, including some demonstrating binucleation.
40x
Pancreas
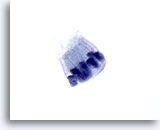
Figure 70
Pancreas FNA, Benign Ductal Cells.
Note the columnar shaped cells arranged in a picket-fence pattern. The nuclear to cytoplasmic ratios remain low. The nuclei are oval in shape with surrounding amphophilic, pale cytoplasm. 60x
Figure 70
Pancreas FNA, Benign Ductal Cells.
Note the columnar shaped cells arranged in a picket-fence pattern. The nuclear to cytoplasmic ratios remain low. The nuclei are oval in shape with surrounding amphophilic, pale cytoplasm.
60x
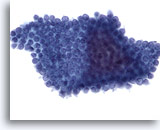
Figure 71
Pancreas FNA, Benign Ductal Cells.
Aspirate of benign ductal cells from the pancreas in which the cells are arranged in a honeycomb fashion with evenly spaced nuclei. 40x
Figure 71
Pancreas FNA, Benign Ductal Cells.
Aspirate of benign ductal cells from the pancreas in which the cells are arranged in a honeycomb fashion with evenly spaced nuclei.
40x
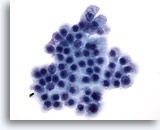
Figure 72
Pancreas FNA, Benign Ductal Cells.
Benign ductal cells in this aspirate arranged in a honeycomb fashion and consisting of uniform cells with round nuclei and well defined cell borders. 60x
Figure 72
Pancreas FNA, Benign Ductal Cells.
Benign ductal cells in this aspirate arranged in a honeycomb fashion and consisting of uniform cells with round nuclei and well defined cell borders.
60x

Figure 73
Pancreas FNA – Skeletal Muscle
Fragment of skeletal muscle is present, which has been inadvertently sampled during a percutaneous aspirate of the pancreas. Bland appearing spindle shaped nuclei are noted. 40x
Figure 73
Pancreas FNA – Skeletal Muscle
Fragment of skeletal muscle is present, which has been inadvertently sampled during a percutaneous aspirate of the pancreas. Bland appearing spindle shaped nuclei are noted.
40x
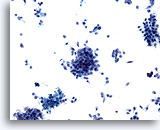
Figure 74
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma consisting of loose clusters of malignant cells, as well as many individually scattered atypical cells. 20x
Figure 74
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma consisting of loose clusters of malignant cells, as well as many individually scattered atypical cells.
20x
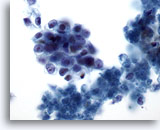
Figure 75
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma in which loose clusters of malignant cells having oval shapes with high nuclear-to-cytoplasmic ratios are noted. Some necrotic debris is present in the background. 60x
Figure 75
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma in which loose clusters of malignant cells having oval shapes with high nuclear-to-cytoplasmic ratios are noted. Some necrotic debris is present in the background.
60x
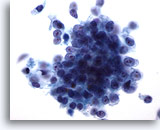
Figure 76
Pancreas FNA, Pancreatic Adenocarcinoma. High power of pancreatic ductal carcinoma consisting of a loose cluster of malignant cells arranged in a syncytial fashion, as well as individually scattered malignant cells. The cells vary from cuboidal to columnar in shape and have high nuclear-to-cytoplasmic ratios. 60x
Figure 76
Pancreas FNA, Pancreatic Adenocarcinoma.
High power of pancreatic ductal carcinoma consisting of a loose cluster of malignant cells arranged in a syncytial fashion, as well as individually scattered malignant cells. The cells vary from cuboidal to columnar in shape and have high nuclear-to-cytoplasmic ratios.
60x
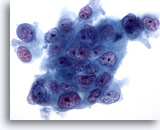
Figure 77
Pancreas FNA, Pancreatic Adenocarcinoma. High power of moderately differentiated pancreatic adenocarcinomas of ductal type consisting of a syncytial arrangement of tumor cells showing marked variation in size and shape. There is also evidence of loss of polarity. Nuclei are hyperchromatic with multiple irregular nucleoli. 60x
Figure 77
Pancreas FNA, Pancreatic Adenocarcinoma.
High power of moderately differentiated pancreatic adenocarcinomas of ductal type consisting of a syncytial arrangement of tumor cells showing marked variation in size and shape. There is also evidence of loss of polarity. Nuclei are hyperchromatic with multiple irregular nucleoli.
60x
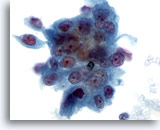
Figure 78
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of moderately differentiated pancreatic carcinoma in which a syncytial cluster of malignant cells is present with considerable variation in nuclear size and shape. 60x
Figure 78
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of moderately differentiated pancreatic carcinoma in which a syncytial cluster of malignant cells is present with considerable variation in nuclear size and shape.
60x
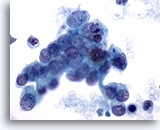
Figure 79
Pancreas FNA, Pancreatic Adenocarcinoma. Loose cluster and individually scattered malignant cells in an aspirate of a pancreatic ductal carcinoma. Tumor cells demonstrate considerable variation in nuclear size and shape. Many of the tumor cells have high nuclear-to-cytoplasmic ratios, as well as irregular chromatin. 60x
Figure 79
Pancreas FNA, Pancreatic Adenocarcinoma.
Loose cluster and individually scattered malignant cells in an aspirate of a pancreatic ductal carcinoma. Tumor cells demonstrate considerable variation in nuclear size and shape. Many of the tumor cells have high nuclear-to-cytoplasmic ratios, as well as irregular chromatin.
60x
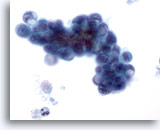
Figure 80
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic ductal carcinoma in which oval shaped malignant cells are present along with some signet ring cells. The large cytoplasmic vacuoles have displaced and deformed the nuclei. 60x
Figure 80
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic ductal carcinoma in which oval shaped malignant cells are present along with some signet ring cells. The large cytoplasmic vacuoles have displaced and deformed the nuclei.
60x
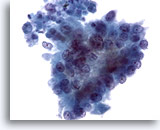
Figure 81
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma in which the tumor cells have a more columnar configuration. Considerable variation in nuclear size and shape is present, as well as loss of polarity. 40x
Figure 81
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma in which the tumor cells have a more columnar configuration. Considerable variation in nuclear size and shape is present, as well as loss of polarity.
40x
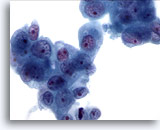
Figure 82
Pancreas FNA, Pancreatic Adenocarcinoma.
Loose clusters of malignant cells having enlarged nuclei with irregular chromatin and prominent nucleoli. Surrounding amphophilic to slightly vacuolated cytoplasm is present. 60x
Figure 82
Pancreas FNA, Pancreatic Adenocarcinoma.
Loose clusters of malignant cells having enlarged nuclei with irregular chromatin and prominent nucleoli. Surrounding amphophilic to slightly vacuolated cytoplasm is present.
60x
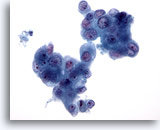
Figure 83
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma in which clusters of malignant cells are present showing considerable variation in nuclear size. Occasional binucleated cells are present. 40x
Figure 83
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of pancreatic adenocarcinoma in which clusters of malignant cells are present showing considerable variation in nuclear size. Occasional binucleated cells are present.
40x
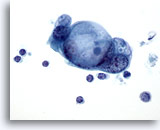
Figure 84
Pancreas FNA, Pancreatic Adenocarcinoma.
A cluster of malignant cells possessing large intracytoplasmic vacuoles having a somewhat targetoid mucin droplet pattern. 60x
Figure 84
Pancreas FNA, Pancreatic Adenocarcinoma.
A cluster of malignant cells possessing large intracytoplasmic vacuoles having a somewhat targetoid mucin droplet pattern.
60x
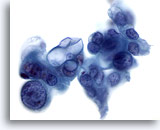
Figure 85
Pancreas FNA, Pancreatic Adenocarcinoma. Aspirate of poorly differentiated adenocarcinoma in which bizarre tumor cells are seen, including some showing considerable variation in nuclear size and shape. In addition, some of the tumor cells have large intracytoplasmic vacuoles. 60x
Figure 85
Pancreas FNA, Pancreatic Adenocarcinoma.
Aspirate of poorly differentiated adenocarcinoma in which bizarre tumor cells are seen, including some showing considerable variation in nuclear size and shape. In addition, some of the tumor cells have large intracytoplasmic vacuoles.
60x
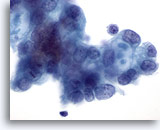
Figure 86
Pancreas FNA, Pancreatic Adenocarcinoma.
Malignant cells including some having targetoid mucin vacuoles. 60x
Figure 86
Pancreas FNA, Pancreatic Adenocarcinoma.
Malignant cells including some having targetoid mucin vacuoles.
60x
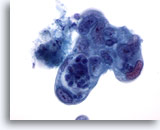
Figure 87
Pancreas FNA, Pancreatic Adenocarcinoma.
Poorly differentiated pancreatic adenocarcinoma showing pleomorphism of the cells with considerable variation of nuclear size and shape and high nuclear-to- cytoplasmic ratios. 60x
Figure 87
Pancreas FNA, Pancreatic Adenocarcinoma.
Poorly differentiated pancreatic adenocarcinoma showing pleomorphism of the cells with considerable variation of nuclear size and shape and high nuclear-to- cytoplasmic ratios.
60x
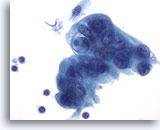
Figure 88
Pancreas FNA, Pancreatic Adenocarcinoma. Poorly differentiated malignant cells including some arranged in a syncytial fashion. The cells have hyperchromatic nuclei with surrounding amphophilic, delicate cytoplasm with frayed borders. One of the tumor cells has a targetoid mucin vacuole. 60x
Figure 88
Pancreas FNA, Pancreatic Adenocarcinoma.
Poorly differentiated malignant cells including some arranged in a syncytial fashion. The cells have hyperchromatic nuclei with surrounding amphophilic, delicate cytoplasm with frayed borders. One of the tumor cells has a targetoid mucin vacuole.
60x
Back to Top
























































































