The Gastrointestinal (GI) tract or the gut is divided into the esophagus, stomach, small intestine and large intestine. They are separated by sphincters that control the passage of contents from one part of the GI tract to the next. In addition, each of these divisions is characterized by a change in the mucosal nature of the lining cells. The GI tract is the digestive organ for the body, a source of immunity generation and an endocrine organ.
Cytologic specimens obtained from the GI tract may be brushings, washings, or Fine Needle Aspiration Biopsies (FNA) under ultrasound guidance. A brushing specimen is usually obtained by passing a brush enclosed inside a transparent sheath through the endoscope and plunging the brush into the lesion five to ten times. The brush is then retracted and the specimen is extruded onto glass slides or into a preservative medium.
The endoscopic FNA involves introducing the needle through a fiberoptic endoscope. When the lesion is localized, negative pressure is applied to the needle and the needle is moved back and forth within the lesion. The pressure is released, the needle is withdrawn and the specimen is collected into a preservative solution.
Normal lining cells of the esophagus consist of non-keratinized squamous epithelial cells. Rarely, metaplastic cells derived from submucosal glands may be seen. Columnar glandular cells may be derived from the stomach, or from Barrett’s esophagus.
Non-specific esophagitis usually shows acute and/or chronic inflammation with reactive changes. Herpetic esophagitis shows classic cytopathic effects consisting of multinucleation, eosinophilic viral inclusions and ground glass nuclei. Candidal esophagitis is diagnosed by the detection of fungal spores and pseudoseptate hyphae. Rarely, other organisms like Aspergillus may be observed.
Cytologic evaluation of the esophagus is an important tool in the diagnosis of esophageal carcinoma, especially in countries with a high incidence of esophageal cancer such as China and Japan, where it is used as a mass screening program. Several authors have showed that a combined cytology -biopsy approach is the most preferred technique for the diagnosis of upper GI neoplasms 1, 2. Glandular dysplasia arising in a background of Barrett’s esophagus typically shows scattered atypical cells with some, but not all, features of adenocarcinoma. Atypical squamous cells with bizarre shapes, hyperchromasia and pleomorphism characterize well-differentiated squamous cell carcinomas. Poorly differentiated squamous cell carcinomas usually show highly pleomorphic cells with high N; C ratio, nucleoli and dense cytoplasm. Adenocarcinomas typically show groups and clusters of neoplastic epithelial cells.
The lining epithelium of the stomach consists of columnar glandular cells usually arranged in honeycomb sheets. Mucin vacuoles may be observed. Parietal and chief cells are rarely seen in gastric brushings. Parietal cells have acidophilic cytoplasm with the pap stain. Chief cells are best identified by Romanowsky stain 3. Helicobacter pylori are gram-negative spiral bacteria easily identified on Pap, Romanowsky, Warthin-Starry and H & E stains. Non-specific reactive and reparative changes, inflammatory cells, mitotic activity, and prominent nucleoli characterize peptic ulcer disease and gastritis. Gastric adenocarcinomas show malignant epithelial cells with pronounced atypia. Signet ring cells may be present. Malignant squamous cells, if present, suggest either adenosquamous carcinoma, the rare pure squamous carcinoma of the stomach or extension of esophageal squamous cell carcinoma into the stomach. Other tumors such as carcinoid and stromal tumors may be rarely diagnosed by gastric brush cytology. However diagnostic sensitivity of these tumors by cytology is far higher by using endoscopic FNA procedure rather than brushing.
Biliary tract brushings are usually acquired through endoscopic retrograde cholangiopancreatography (ERCP). Specimens can also be obtained from biliary stents. The main indication for biliary cytology is suspected malignancy in a patient with a biliary stricture. Reactive and reparative changes are frequently seen with infectious diseases, and primary sclerosing cholangitis (PSC). Dysplasia may be observed in the biliary tract. Cytologic features include crowding and overlapping, increased nuclear to cytoplasmic ratio and abnormal chromatin distribution. The atypia, however, is less severe than in adenocarcinoma. Adenocarcinoma in the biliary tract (cholangiocarcinoma) is cytologically similar to those seen in the GI tract. The mucinous variant can be especially difficult to diagnose due to its bland cytologic features. These cells contain abundant mucin and can be sometimes mistaken for histiocytes.
Cells may be obtained by endoscopic brushing, washing or by FNA. Normal colonic mucosa is represented by tall columnar cells arranged in sheets or singly. Goblet cells may be seen. Some authors have described cytologic findings of adenoma wherein tubular adenoma has more flat and blunted cells while villous adenoma has elongated and spindly cells 4. Adenocarcinoma of the colon shows cohesive, highly atypical groups of glandular cells with prominent nucleoli and a necrotic background. The sensitivity of colonic brushing in the diagnosis of colon carcinoma ranges from 70 to 85 % 5. Combining cytology with biopsy however, yielded best accuracy 6.
Much like the transformation zone of the cervix, the squamocolumnar junction of the anal canal is prone to the development of neoplasia 7. The cytologic appearance of the two sample types is very similar. In fact, findings may be classified according to the criteria defined by the Bethesda System 8.
Cytologic material for evaluation is easily obtained by directly scraping the area and smearing it on glass slides or rinsing into a preservative. In addition, anal “pap” smears are being increasingly employed as a screening tool to assess dysplastic changes, especially in the HIV positive population 9. Squamous cell carcinomas of the anal region show characteristic neoplastic squamous cells with varying degrees of differentiation.
Reminder: You may click on any slide image
for an enlarged view.
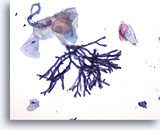
Figure 1
Esophageal brush – Aspergillus
Esophageal brush demonstrating Aspergillus species with septate hyphae, and 45 degree angle branching. Rarely, associated squamous cell atypia may be observed. 20x
Figure 1
Esophageal brush – Aspergillus
Esophageal brush demonstrating Aspergillus species with septate hyphae, and 45 degree angle branching. Rarely, associated squamous cell atypia may be observed.
20x
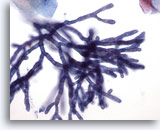
Figure 2
Esophageal brush – Aspergillus
Septae and 45-degree angle branching are well seen on higher power. 40x
Figure 2
Esophageal brush – Aspergillus
Septae and 45-degree angle branching are well seen on higher power.
40x
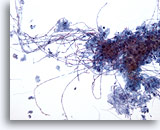
Figure 3
Esophageal brush – Candida
Candidal esophagitis is characterized by identifying elongated pseudohyphal forms and ovoid yeast forms. 20x
Figure 3
Esophageal brush – Candida
Candidal esophagitis is characterized by identifying elongated pseudohyphal forms and ovoid yeast forms.
20x
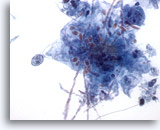
Figure 4
Esophageal brush – Candida
Budding yeast forms are easily seen. 60x
Figure 4
Esophageal brush – Candida
Budding yeast forms are easily seen.
60x
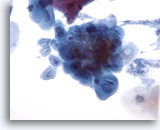
Figure 5
Esophageal brush – Herpes
Herpetic infection is demonstrated by classic cytologic features including multinucleation, molding of nuclei, and ground glass chromatin. 40x
Figure 5
Esophageal brush – Herpes
Herpetic infection is demonstrated by classic cytologic features including multinucleation, molding of nuclei, and ground glass chromatin.
40x

Figure 6
Esophageal brush – Herpes
Eosinophilic intranuclear inclusions can be observed in Herpes infection. 40x
Figure 6
Esophageal brush – Herpes
Eosinophilic intranuclear inclusions can be observed in Herpes infection.
40x
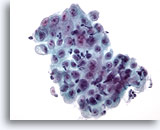
Figure 7
Esophageal brush – Ulcer
Sheet of epithelial cells with overlying inflammatory cells and reactive changes. 40x
Figure 7
Esophageal brush – Ulcer
Sheet of epithelial cells with overlying inflammatory cells and reactive changes.
40x
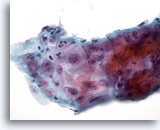
Figure 8
Esophageal brush – Ulcer
Atypical repair in esophageal ulcer showing inflammatory infiltrate, reactive atypia with prominent nucleoli, smooth nuclear membranes and fine chromatin. 60x
Figure 8
Esophageal brush – Ulcer
Atypical repair in esophageal ulcer showing inflammatory infiltrate, reactive atypia with prominent nucleoli, smooth nuclear membranes and fine chromatin.
60x
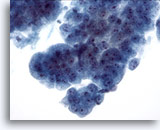
Figure 9
Esophageal brush, High-grade dysplasia in Barrett’s epithelium.
Cohesive groups of atypical glandular cells with some but not all features of adenocarcinoma. 40x
Figure 9
Esophageal brush, High-grade dysplasia in Barrett’s epithelium.
Cohesive groups of atypical glandular cells with some but not all features of adenocarcinoma.
40x
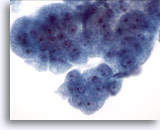
Figure 10
Esophageal brush, High-grade dysplasia in Barrett’s epithelium.
A group of cohesive cells with variably sized nuclei, high nuclear to cytoplasmic ratio, overlapping and crowding. Cytologic features fall short of a definitive diagnosis of adenocarcinoma. 60x
Figure 10
Esophageal brush, High-grade dysplasia in Barrett’s epithelium.
A group of cohesive cells with variably sized nuclei, high nuclear to cytoplasmic ratio, overlapping and crowding. Cytologic features fall short of a definitive diagnosis of adenocarcinoma.
60x
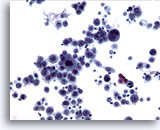
Figure 11
Esophageal brush, Squamous cell carcinoma.
Highly atypical epithelial cells diagnostic of poorly differentiated carcinoma. 20x
Figure 11
Esophageal brush, Squamous cell carcinoma.
Highly atypical epithelial cells diagnostic of poorly differentiated carcinoma. 20x
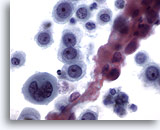
Figure 12
Esophageal brush, Poorly differentiated squamous cell carcinoma.
Atypical epithelial cells with abnormal chromatin. Squamous differentiation is not obvious. 60x
Figure 12
Esophageal brush, Poorly differentiated squamous cell carcinoma.
Atypical epithelial cells with abnormal chromatin. Squamous differentiation is not obvious.
60x
Gastric Cytology
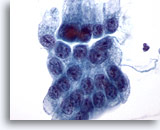
Figure 13
Gastric brush, Gastric dysplasia.
Flat sheet of atypical gastric epithelial cells with enlarged nuclei, crowding, and focal overlapping of cells. 60x
Figure 13
Gastric brush, Gastric dysplasia.
Flat sheet of atypical gastric epithelial cells with enlarged nuclei, crowding, and focal overlapping of cells.
60x
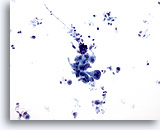
Figure 14
Gastric brush, Gastric adenocarcinoma.
Cohesive group of atypical cells. Note the dirty background. 20x
Figure 14
Gastric brush, Gastric adenocarcinoma.
Cohesive group of atypical cells. Note the dirty background.
20x
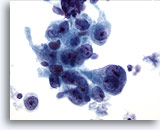
Figure 15
Gastric brush, Gastric adenocarcinoma.
Neoplastic epithelial cells demonstrating pleomorphism, and prominent nucleoli. 60x
Figure 15
Gastric brush, Gastric adenocarcinoma.
Neoplastic epithelial cells demonstrating pleomorphism, and prominent nucleoli.
60x
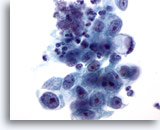
Figure 16
Gastric brush, Gastric adenocarcinoma.
Adenocarcinoma with a signet ring cell at one end. Signet ring cells are characterized by huge mucin vacuoles that compress the nucleus into a crescent against the nuclear membrane. 60x
Figure 16
Gastric brush, Gastric adenocarcinoma.
Adenocarcinoma with a signet ring cell at one end. Signet ring cells are characterized by huge mucin vacuoles that compress the nucleus into a crescent against the nuclear membrane.
60x
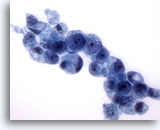
Figure 17
Gastric brush, Gastric adenocarcinoma.
More signet ring cells with the typical morphology. 60x
Figure 17
Gastric brush, Gastric adenocarcinoma.
More signet ring cells with the typical morphology.
60x
Colonic Cytology
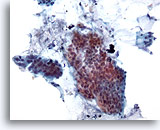
Figure 18
Colonic brush, Colonic adenocarcinoma.
Colonic brushing demonstrating atypical epithelial cells under low power. 20x
Figure 18
Colonic brush, Colonic adenocarcinoma.
Colonic brushing demonstrating atypical epithelial cells under low power. 20x
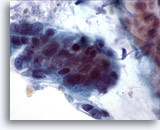
Figure 19
Colonic brush, Colonic adenocarcinoma.
High power showing pleomorphism, crowding and overlapping of cells. 60x
Figure 19
Colonic brush, Colonic adenocarcinoma.
High power showing pleomorphism, crowding and overlapping of cells.
60x
Pancreatic Cytology
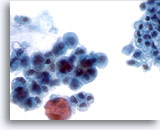
Figure 20
Pancreatic duct brush, Pancreatic adenocarcinoma.
Pancreatic brushing demonstrating cytologic features of adenocarcinoma of pancreas. Compare with adjacent non-neoplastic epithelium. 40x
Figure 20
Pancreatic duct brush, Pancreatic adenocarcinoma.
Pancreatic brushing demonstrating cytologic features of adenocarcinoma of pancreas. Compare with adjacent non-neoplastic epithelium.
40x
Bile Duct Cytology
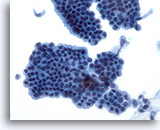
Figure 21
Bile duct brush, Normal bile duct epithelium.
Honeycomb sheet of epithelial cells without atypia. 40x
Figure 21
Bile duct brush, Normal bile duct epithelium.
Honeycomb sheet of epithelial cells without atypia.
40x

Figure 22
Bile duct brush, Cholangiocarcinoma.
Malignant epithelial cells adjacent to normal bile duct epithelium. 20x
Figure 22
Bile duct brush, Cholangiocarcinoma.
Malignant epithelial cells adjacent to normal bile duct epithelium.
20x
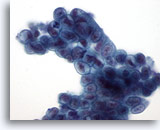
Figure 23
Bile duct brush, Well-differentiated mucinous adenocarcinoma.
Syncytial group of mucinous epithelium with nuclear hyperchromasia, and irregular nuclear membranes. 60x
Figure 23
Bile duct brush, Well-differentiated mucinous adenocarcinoma.
Syncytial group of mucinous epithelium with nuclear hyperchromasia, and irregular nuclear membranes.
60x
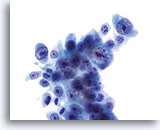
Figure 24
Bile duct brush, Cholangiocarcinoma.
Poorly differentiated adenocarcinoma with severe nuclear atypia and prominent nucleoli. 60x
Figure 24
Bile duct brush, Cholangiocarcinoma.
Poorly differentiated adenocarcinoma with severe nuclear atypia and prominent nucleoli.
60x
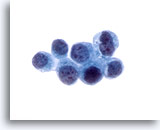
Figure 25
Bile duct brush, Metastatic breast carcinoma.
Adenocarcinoma metastatic from the breast. Diagnosis was confirmed using immunohistochemical stains on the cellblock. 60x
Figure 25
Bile duct brush, Metastatic breast carcinoma.
Adenocarcinoma metastatic from the breast. Diagnosis was confirmed using immunohistochemical stains on the cellblock.
60x
Anal Cytology
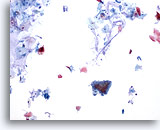
Figure 26
Anal brush, Normal glandular epithelium.
Anal brushings routinely contain background mucin, normal glandular and normal squamous epithelium. Hyperkeratosis is typically abundant. 10x
Figure 26
Anal brush, Normal glandular epithelium.
Anal brushings routinely contain background mucin, normal glandular and normal squamous epithelium. Hyperkeratosis is typically abundant.
10x
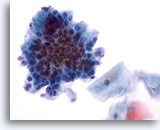
Figure 27
Anal brush, Normal glandular epithelium.
At higher power the cobblestone and picket fence morphology is apparent. 40x
Figure 27
Anal brush, Normal glandular epithelium.
At higher power the cobblestone and picket fence morphology is apparent.
40x

Figure 28
Anal brush, Atypical squamous cells (ASC).
Changes suggestive of HPV but not sufficient for a LSIL diagnosis fall in the ASC category. 40x
Figure 28
Anal brush, Atypical squamous cells (ASC).
Changes suggestive of HPV but not sufficient for a LSIL diagnosis fall in the ASC category.
40x
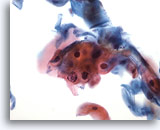
Figure 29
Anal brush, Atypical squamous cells (ASC).
Atypical parakeratosis (pk), not diagnostic of LSIL, falls into the category of ASC. 40x
Figure 29
Anal brush, Atypical squamous cells (ASC).
Atypical parakeratosis (pk), not diagnostic of LSIL, falls into the category of ASC.
40x
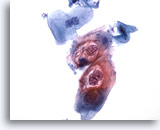
Figure 30
Anal brush, Anal Intraepithelial Lesion, Low Grade.
These cells exhibit the binucleation and cytoplasmic cavitations associated with HPV infection and are diagnostic of a low-grade anal intraepithelial lesion. 40x
Figure 30
Anal brush, Anal Intraepithelial Lesion, Low Grade.
These cells exhibit the binucleation and cytoplasmic cavitations associated with HPV infection and are diagnostic of a low-grade anal intraepithelial lesion.
40x

Figure 31
Anal brush, Anal Intraepithelial Lesion, Low Grade.
Squamous metaplastic cells with enlarged nuclei may characterize low grade anal intraepithelial lesions. 40x
Figure 31
Anal brush, Anal Intraepithelial Lesion, Low Grade.
Squamous metaplastic cells with enlarged nuclei may characterize low grade anal intraepithelial lesions.
40x
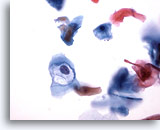
Figure 32
Anal brush, Anal Intraepithelial Lesion, Low Grade.
Cavitation caused by HPV is diagnostic of low grade anal intraepithelial lesions. 40x
Figure 32
Anal brush, Anal Intraepithelial Lesion, Low Grade.
Cavitation caused by HPV is diagnostic of low grade anal intraepithelial lesions.
40x
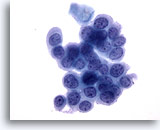
Figure 33
Anal brush, Anal Intraepithelial Lesion, High Grade.
Round cells with high N/C ratios and evenly distributed chromatin display the cytologic features diagnostic of high-grade anal intraepithelial lesion. 40x
Figure 33
Anal brush, Anal Intraepithelial Lesion, High Grade.
Round cells with high N/C ratios and evenly distributed chromatin display the cytologic features diagnostic of high-grade anal intraepithelial lesion.
40x
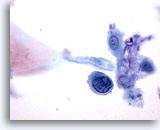
Figure 34
Anal brush, Anal Intraepithelial Lesion, High Grade.
Single cells with high N/C ratios and hyperchromatic nuclei are diagnostic of high grade anal intraepithelial lesions. 60x
Figure 34
Anal brush, Anal Intraepithelial Lesion, High Grade.
Single cells with high N/C ratios and hyperchromatic nuclei are diagnostic of high grade anal intraepithelial lesions.
60x
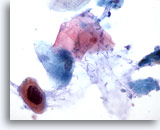
Figure 35
Anal brush, Anal Intraepithelial Lesion, High Grade.
Atypical keratinized cells with enlarged, hyperchromatic nuclei are sometimes seen in high grade anal intraepithelial lesions. 60x
Figure 35
Anal brush, Anal Intraepithelial Lesion, High Grade.
Atypical keratinized cells with enlarged, hyperchromatic nuclei are sometimes seen in high grade anal intraepithelial lesions.
60x



































