Epithelial Cell Abnormalities
GLANDULAR CELLS
Although from the lay perspective the principle intent of the Pap test is the detection of precursors of squamous cancer, cytologists are well aware of the opportunities and challenges posed by glandular cells in the cervical sample. This has become particularly apparent in recent years, when the limitations of the Pap test as a method for detecting glandular lesions of the cervix have become evident. Numerous studies have described the low sensitivity of the Pap test for the detection of adenocarcinoma.
Sensitivity of Pap testing for true glandular neoplasia is less than that for squamous lesions, in part because glandular lesions arise in the endocervical canal where they may be difficult to sample. (See Suggested Readings) The specificity of cytologic detection of glandular abnormalities is also problematic, given the high frequency in which cytologic diagnosis of “Atypical Glandular Cells (NOS)” is subsequently associated with non-glandular histology, such as a squamous lesion or a reactive process on follow-up. This difficulty arises from several factors. The sampling technique can impose artifacts on glandular cell clusters that make their identification and classification difficult. Smearing and air-drying, or rounding-up of cells in fluid all affect morphology. Also, glandular cells in Pap samples can have diverse origins, from the endocervix, lower uterine segment, endometrium, or other Muellerian tissues. This diversity, coupled with the relative infrequency of glandular lesions, represents a challenge to the cytologist.

Reminder: You may click on any slide image
for an enlarged view.
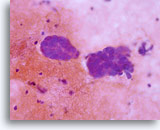
Conventional Pap smear
Adenocarcinoma
Conventional Pap smear
Adenocarcinoma
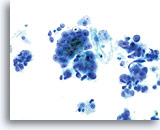
ThinPrep Pap Test
Adenocarcinoma
ThinPrep Pap Test
Adenocarcinoma
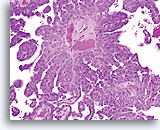
Biopsy
Papillary serous carcinoma of the endometrium
Same Patient, Split Sample
Biopsy
Papillary serous carcinoma of the endometrium
Same Patient, Split Sample
Endometrial Cells: Other
According to The Bethesda System 2014; Third Edition, the presence of endometrial cells in a woman 45 years or older, even when benign in appearance, should be reported, because these women are believed to be at higher risk of having or developing endometrial disease. The “Endometrial Cells: The How and When of Reporting” chapter illustrates normal endometrial glandular and stromal cell components.
Atypical Glandular Cells (NOS)
Joyce E. Johnson, MD
The atypical category for glandular cells (NOS) refers to glandular cells that have nuclear enlargement, crowding, slight hyperchromasia, and possible chromocenters/nucleoli and/or architectural abnormalities that distinguish them from their normal counterparts. These changes, however, are not sufficient to support an unequivocal diagnosis of adenocarcinoma.
The Bethesda System guidelines recommend that an interpretation of atypical glandular cells be qualified, when possible, to indicate whether the cells of origin are endometrial or endocervical. The accuracy of this distinction, and the determination of the presence or absence of atypia, may be enhanced by collection of the cervical sample using the ThinPrep® Pap Test. The ThinPrep Pap Test, through the rapid fixation of the cervical material collected, eliminates the air-drying artifact that may otherwise hinder a qualifying diagnosis in cases of atypical glandular cells. Several laboratories have reported a decreased atypical glandular cells (NOS) rate, with a higher rate of abnormal histology, after implementing the ThinPrep Pap Test. (See Suggested Readings 1, 5, and 6 ) This is a result of proper classification of some atypical glandular cells (NOS) cases as HSIL, glandular neoplasia and/or reactive endocervical cells.
Normal endocervical and endometrial cells are well preserved and maintain their classic morphologic features on a ThinPrep preparation, as described in the Negative for Intraepithelial Lesion or Malignancy Chapter. The fluid-based fixation method also may help in accurately classifying other entities such as tubal metaplasia and cells of true lower uterine segment, adenocarcinoma in situ (AIS), and squamous lesions involving glands. The smearing technique employed with the conventional Pap smear can be harsh and destructive to cellular material, making the identification of cilia difficult. With the ThinPrep Pap Test, the rinsing technique and gentle collection and cell transfer utilized by the ThinPrep System enhances the preservation and ultimately the visualization of the fragile cilia which permits recognizing tubal metaplasia.
Atypical Endometrial Cells (NOS)
Experience and biopsy correlation studies with the ThinPrep Pap Test have shown that cases containing endometrial cells called “atypical NOS” most often reflect the presence of endometrial hyperplasia or proliferative endometrium in peri- and postmenopausal women. These entities may be cytologically impossible to differentiate from benign, atypical and neoplastic endometrial processes. The endometrial cells may be cytologically bland, but because of the increased risk conferred by the patient’s age and menopausal status, such cells must be reported and correlated clinically. Alternatively, the cells may exhibit atypia beyond the range of normal for the patient’s age and history, but a diagnosis of adenocarcinoma is not a consideration. The Pap test has not proven to be a reliable screening procedure for detection of endometrial disease, supporting a conservative approach to the diagnosis of endometrial cells.
Atypical endometrial cells (NOS) is most commonly used when endometrial cells are present in a peri- or post-menopausal patient not on hormone replacement therapy. These endometrial cells may appear cytologically benign (categorize as “Other”) or present with atypical features, such as cell enlargement, more abundant cytoplasm, enlargement and rounding up of the nucleus, prominent nucleoli and multiple discrete cytoplasmic vacuoles. Cell groups will typically maintain a 3-dimensional presentation, but groups may be larger than normal and present with “scalloping” around the edges. Single endometrial cells may also be present. Any endometrial cells present in a woman 45 years of age or older should be reported in the “Other” category. If these endometrials appear atypical they may be placed in the atypical endometrial cells (NOS) category if not convincingly malignant.
Criteria for a diagnosis of atypical endometrial cells (NOS) may include the following:
Reminder: You may click on any slide image
for an enlarged view.
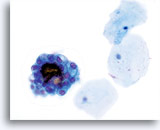
Atypical endometrial cells (NOS)
(51 yo patient, peri-menopausal) This 3-dimensional group of small glandular cells with abundant cytoplasm has multiple distinct vacuoles. Cells have rounded up and nuclei are round to oval with distinct chromatin patterns.
40x
Biopsy – Benign proliferative endometrium
Atypical endometrial cells (NOS)
(51 yo patient, peri-menopausal) This 3-dimensional group of small glandular cells with abundant cytoplasm has multiple distinct vacuoles. Cells have rounded up and nuclei are round to oval with distinct chromatin patterns.
40x
Biopsy – Benign proliferative endometrium
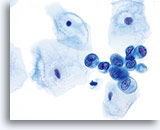
Atypical endometrial cells (NOS)
(51 yo patient, LMP unknown) Pictured is a loose aggregate of cells with increased N/C ratio, irregular nuclear membranes and coarse, somewhat cleared chromatin.
60x
Biopsy – Benign proliferative endometrium
Atypical endometrial cells (NOS)
(51 yo patient, LMP unknown) Pictured is a loose aggregate of cells with increased N/C ratio, irregular nuclear membranes and coarse, somewhat cleared chromatin.
60x
Biopsy – Benign proliferative endometrium
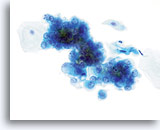
Atypical endometrial cells (NOS)
(51 yo patient, LMP unknown) A dyshesive 3-dimensional group of glandular cells with enlarged, round to oval nuclei, smooth nuclear membranes and cleared chromatin is noted.
60x
Biopsy – Benign proliferative endometrium
Atypical endometrial cells (NOS)
(51 yo patient, LMP unknown) A dyshesive 3-dimensional group of glandular cells with enlarged, round to oval nuclei, smooth nuclear membranes and cleared chromatin is noted.
60x
Biopsy – Benign proliferative endometrium
Atypical Endocervical Cells (NOS)
Experience has shown that the term atypical endocervical cells (NOS) is most often used when the morphology of a conventional Pap smear is compromised by air drying, thick groups and smearing artifact. The ThinPrep® Pap Test minimizes these types of limiting factors, allowing the cytologist to more easily visualize the endocervical cells and assess them. With air drying eliminated, the nuclei are no longer “blown-up” beyond their normal range allowing better visualization of N/C ratios, chromatin pattern and other nuclear features for more accurate distinction of normal from abnormal.
In normal endocervical cells, nucleoli are commonly present, reflecting the constant activity of the endocervical component. In the resting state, these nucleoli are small and regular and can be multiple. When endocervical cells are subject to stimulation or undergoing repair, they may acquire some abnormal features, but not to a degree to support a diagnosis of atypical endocervical cells, favor neoplastic. Cases of abnormal repair may be categorized as atypical endocervical cells (NOS). As indicated previously, nucleoli may be prominent and prevalent, which prompts cytotechnologists to diagnose atypical endocervical cells (NOS). As with all diagnoses, sufficient criteria must be present to render a diagnosis of atypia and the presence of nucleoli alone is not sufficient. Additional nuclear abnormalities and a disruption of the “normal” tissue architecture are required.
Criteria for a diagnosis of atypical endocervical cells (NOS), may include:
Reminder: You may click on any slide image
for an enlarged view.
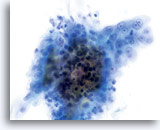
Atypical endocervical cells (NOS)
Pictured is a sheet of endocervical cells with slightly disordered architecture, slight depth of focus to group, abundant cytoplasm, uniform, bland chromatin pattern and prominent nucleoli in each nucleus. The diagnosis of atypia may arise due to depth of focus to the group and slightly disordered architecture.
60X
Biopsy – Inflammatory Atypia
Atypical endocervical cells (NOS)
Pictured is a sheet of endocervical cells with slightly disordered architecture, slight depth of focus to group, abundant cytoplasm, uniform, bland chromatin pattern and prominent nucleoli in each nucleus. The diagnosis of atypia may arise due to depth of focus to the group and slightly disordered architecture.
60X
Biopsy – Inflammatory Atypia
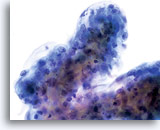
Atypical endocervical cells (NOS)
The specimen contains a rounded up (3-dimensional) group of endocervical cells with slight disruption to architecture. Nuclei are all similar in size and nuclear membranes are smooth to slightly irregular.
60x
Biopsy – Chronic Inflammation
Atypical endocervical cells (NOS)
The specimen contains a rounded up (3-dimensional) group of endocervical cells with slight disruption to architecture. Nuclei are all similar in size and nuclear membranes are smooth to slightly irregular.
60x
Biopsy – Chronic Inflammation
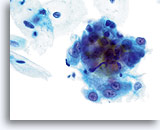
Atypical endocervical cells (NOS)
A loose grouping of endocervical cells exhibits an apparent loss of polarity. Nuclei are round to oval with slight nuclear membrane irregularities and slight crowding. The chromatin is granular but evenly distributed and prominent nucleoli are present.
60x
Biopsy – Chronic Inflammation
Atypical endocervical cells (NOS)
A loose grouping of endocervical cells exhibits an apparent loss of polarity. Nuclei are round to oval with slight nuclear membrane irregularities and slight crowding. The chromatin is granular but evenly distributed and prominent nucleoli are present.
60x
Biopsy – Chronic Inflammation
Atypical Endocervical Cells, Favor Neoplastic
This diagnostic category lies in-between atypical endocervical cells (NOS) and a definitive diagnosis of adenocarcinoma in situ (AIS) and may very well overlap into well-differentiated adenocarcinoma of the cervix. (no photographs)
Adenocarcinoma In Situ (AIS)
The cells of adenocarcinoma in situ on ThinPrep slides are well visualized and the cytotechnologist is often alerted to this possibility on screening with low power. When the lesion has been adequately sampled, the preparation contains variably sized “dark” groups of cells with hyperchromatic nuclei and little cytoplasm. The groups of cells are often larger than is typical with normal endocervical components, reflecting an abnormal growth pattern and dishesion within the epithelial tissue. This presentation warrants a closer look at these groups.
Reminder: You may click on any slide image
for an enlarged view.
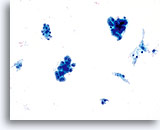
Adenocarcinoma in situ
Variably sized, crowded, hyperchromatic groups of cells are seen on screening power which warrant a closer inspection. 20x
Adenocarcinoma in situ
Variably sized, crowded, hyperchromatic groups of cells are seen on screening power which warrant a closer inspection. 20x
On higher power, these groups/sheets of cells may exhibit abnormal cytologic and architectural features, including cell crowding, stratification, rosette formation, feathering, hyperchromasia, variable nucleoli, and nuclear membrane irregularities. The most consistent and striking feature is cellular crowding, where the nuclei are elongate and “mold” against one another to accommodate the high rate of the cell division. This also causes the nuclei to stratify. Nuclei can often be seen throughout the entire thickness of the tissue fragment. Feathering is also present, though it may be more subtle than on the conventional Pap smear. Feathering is exaggerated by the smearing of cells on the conventional Pap smear. In the ThinPrep® Pap Test the collection and transfer techniques are much more gentle. However, feathering is inherent to the dishesion of the tissue and appears in the ThinPrep® Pap Test as cytoplasmic “nipples” and nuclei falling off the tissue fragment. Because cells can appear to be separating from the parent tissue fragment in benign conditions, this criterion should not be used alone. Appropriate nuclear features are required for an AIS diagnosis.
Criteria for a diagnosis of AIS:
Reminder: You may click on any slide image
for an enlarged view.
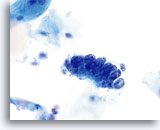
AIS
Endocervical cells presenting in a strip with pronounced nuclear crowding.
60x
Biopsy – AIS
AIS
Endocervical cells presenting in a strip with pronounced nuclear crowding.
60x
Biopsy
AIS
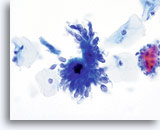
AIS
Endocervical cells presenting in a pseudo-rosette formation and exhibiting “feathering” and nuclear elongation due to crowding.
60x
Biopsy
AIS
AIS
Endocervical cells presenting in a pseudo-rosette formation and exhibiting “feathering” and nuclear elongation due to crowding.
60x
Biopsy
AIS
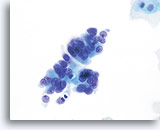
AIS
A strip of endocervical cells exhibiting pseudostratification and finely vacuolated cytoplasm with indistinct cytoplasmic borders. Architecture is disrupted although cells are still attempting to maintain a glandular configuration. Note increased N/C ratio and the variable presence of nucleoli.
60x
Biopsy – AIS
AIS
A strip of endocervical cells exhibiting pseudostratification and finely vacuolated cytoplasm with indistinct cytoplasmic borders. Architecture is disrupted although cells are still attempting to maintain a glandular configuration. Note increased N/C ratio and the variable presence of nucleoli.
60x
Biopsy
AIS
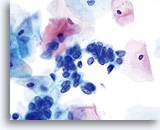
AIS
High N/C ratios are evident in nuclei that take up at least 2/3 of the cytoplasm. Chromatin is coarse but evenly distributed and micronucleoli are present.
60x
Biopsy – AIS
AIS
High N/C ratios are evident in nuclei that take up at least 2/3 of the cytoplasm. Chromatin is coarse but evenly distributed and micronucleoli are present.
60x
Biopsy
AIS
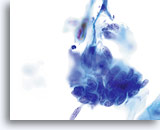
AIS
Nuclear elongation is clearly evident in this group of atypical endocervical cells. Also noted is loss of nuclear polarity, loss of normal architecture, nuclear crowding, and “molding” of the nuclei. Note the flat nuclear membranes where they push up against each other (a sign of true crowding).
60x
Biopsy – AIS
AIS
Nuclear elongation is clearly evident in this group of atypical endocervical cells. Also noted is loss of nuclear polarity, loss of normal architecture, nuclear crowding, and “molding” of the nuclei. Note the flat nuclear membranes where they push up against each other (a sign of true crowding).
60x
Biopsy
AIS
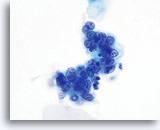
AIS
Nuclear membranes can be smooth to markedly irregular. This image exhibits nuclear membranes that are thickened and moderately irregular. Chromatin is coarse and nucleoli are prominent.
60x
Biopsy – AIS
AIS
Nuclear membranes can be smooth to markedly irregular. This image exhibits nuclear membranes that are thickened and moderately irregular. Chromatin is coarse and nucleoli are prominent.
60x
Biopsy
AIS
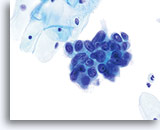
AIS
Nucleoli can be indistinct to prominent. In this image, the nucleoli are prominent and present in a majority of the nuclei. Rapid fixation makes the presence of nucleoli another important piece of criteria in the diagnosis of AIS.
60x
Biopsy – AIS
AIS
Nucleoli can be indistinct to prominent. In this image, the nucleoli are prominent and present in a majority of the nuclei. Rapid fixation makes the presence of nucleoli another important piece of criteria in the diagnosis of AIS.
60x
Biopsy
AIS
Summary of Atypical Glandular Cells Criteria:
|
ATYPICAL ENDOMETRIAL CELLS (NOS) |
ATYPICAL ENDOCERVICAL CELLS (NOS) |
ENDOCERVICAL ADENOCARCINOMA IN SITU |
| Cell Type |
Endometrial |
Endocervical |
Endocervical |
| Tissue Presentation |
3-dimensional; loose aggregates; single cells |
Flat sheets; well organized; “school of fish” arrangement |
Strips of cells; rosette formation; feathering; crowding; stratification |
| Cytoplasm |
More abundant than benign; finely vacuolated with multiple distinct vacuoles |
Abundant; “tissue culture” appearance |
Scant; finely vacuolated; indistinct cytoplasmic borders |
| N/C Ratio |
High |
Low |
High; nucleus takes up 2/3 of the cytoplasm |
| Nucleus |
Oval to round |
Oval to round |
Oval to elongated |
| Nuclear Membrane |
Irregular to Smooth |
Smooth; possible slight irregularities; possibly slightly thickened |
Smooth to markedly irregular; notching; thickened |
| Chromatin |
Coarse with chromatin clearing; may vary from nucleus to nucleus; hyperchromatic |
Granular; evenly distributed; uniformly hypochromatic |
Coarse to finely granular; evenly distributed; hyperchromatic |
| Nucleoli |
Absent or small single nucleoli |
In every nucleus; single to multiple; small to moderate |
Indistinct to prominent; variably present |
LOOK-ALIKE ENTITIES
The more common look-alike entities observed on the conventional Pap smear are more easily identified, and become less of a diagnostic difficulty, with the ThinPrep Pap Test. Because of rapid fixation and gentle cell transfer techniques, entities such as tubal metaplasia, lower uterine segment cells and repair are more apparent.
|
ENDOCERVICAL REPAIR |
TUBAL METAPLASIA |
LOWER UTERINE SEGMENT |
ATYPICAL ENDOCERVICAL CELLS, FAVOR NEOPLASIA OR AIS |
| Cell Type |
Endocervical cells |
Small cuboidal fallopian tube- type glandular cells |
Endometrial cells |
Endocervical cells |
| Tissue Presentation |
Flat, loose sheet |
Crowded honeycomb presentation |
Honeycomb presentation with overlapping nuclei, often in large sheets |
Strips of tissue with nuclear crowding, stratification and feathering |
| Unique Feature |
“School of fish” tissue appearance; low N/C ratio; prominent nucleoli |
Terminal bars and cilia |
Very small cells with scant cytoplasm and uniform appearance |
Nuclear crowding and stratification; no cilia or terminal bars identified |
Reminder: You may click on any slide image
for an enlarged view.
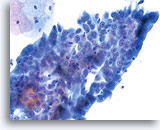
Look-alike
Repair
Note the flatness of this sheet of reparative cells. All of the nuclei are in the same plane and lack the nuclear crowding characteristic of AIS. Nuclei are uniformly spaced and round to oval, rather than elongate. 40x
Look-alike
Repair
Note the flatness of this sheet of reparative cells. All of the nuclei are in the same plane and lack the nuclear crowding characteristic of AIS. Nuclei are uniformly spaced and round to oval, rather than elongate.
40x
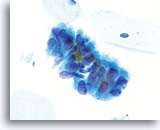
Look-alike – Tubal metaplasia
Tubal metaplasia can closely mimic AIS. However, on close inspection the abnormalities, ie: crowding, nuclear elongation, irregular nuclear membranes and abnormal tissue fragments are less severe. Locating cilia and/or terminal bars confirms benignancy. 60X
Look-alike
Tubal metaplasia
Tubal metaplasia can closely mimic AIS. However, on close inspection the abnormalities, ie: crowding, nuclear elongation, irregular nuclear membranes and abnormal tissue fragments are less severe. Locating cilia and/or terminal bars confirms benignancy.
60X
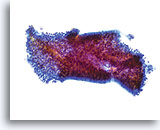
Look-alike – Lower uterine segment.
This tissue fragment of lower uterine segment is composed of small, cuboidal cells with uniform nuclei and bland chromatin. At low power the cells appear crowded; however on closer inspection they are simply tightly packed. The cohesiveness and uniformity of such fragments at low power are the keys to diagnosis. A stromal component is usually present as well and is an aid in diagnosis. 20x
Look-alike
Lower uterine segment
This tissue fragment of lower uterine segment is composed of small, cuboidal cells with uniform nuclei and bland chromatin. At low power the cells appear crowded; however on closer inspection they are simply tightly packed. The cohesiveness and uniformity of such fragments at low power are the keys to diagnosis. A stromal component is usually present as well and is an aid in diagnosis.
20x
An important AIS look-alike is a squamous lesion involving the endocervical gland space. On the ThinPrep Pap Test, this entity is more frequently correctly identified as a squamous lesion, leading to the misconception that the laboratory is “missing” cases of atypical glandular cells. On the contrary, publications have shown that when using the ThinPrep Pap Test in clinical practice, an atypical glandular cell diagnosis is shown to be more sensitive, and more specific in its biopsy correlation. (See Suggested Readings) In summary, when the ThinPrep process is used, squamous lesions involving the endocervical glands are more frequently identified as squamous and “Atypical Glandular Cells” are, on biopsy, more often associated with a glandular lesion.
The first and most notable criterion beneficial in distinguishing AIS from a squamous lesion involving the endocervical glands is the architecture of the cell group(s). AIS presents with truly glandular architecture: strips, rosettes, pseudostratification, etc. Squamous lesions will occur in flatter sheets composed of cells with more cytoplasm, more dyshesion, and with nuclei lacking polarity, but not crowded. Nuclear elongation is therefore absent.
Reminder: You may click on any slide image
for an enlarged view.
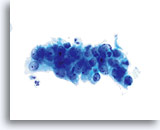
Look-alike
AIS
This crowded, stratified group of glandular cells comes from a case of AIS. Note the basal nuclei piling up from the lower layer of the epithelium. 60x
Look-alike
AIS
This crowded, stratified group of glandular cells comes from a case of AIS. Note the basal nuclei piling up from the lower layer of the epithelium.
60x
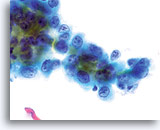
Look-alike
Squamous cell carcinoma in glands
A flat sheet of malignant squamous cells originated from a squamous lesion invading the endocervical gland space. Note the centrally placed nuclei and dense, round squamous cytoplasm. 60x
Look-alike
Squamous cell carcinoma in glands
A flat sheet of malignant squamous cells originated from a squamous lesion invading the endocervical gland space. Note the centrally placed nuclei and dense, round squamous cytoplasm.
60x
SUGGESTED READINGS:
- Ashfaq R et al: ThinPrep Pap Test accuracy for glandular disease. Acta Cytol 1999;43: 81-85.
- Bai H et al: ThinPrep® Pap Test promotes detection of glandular lesions of the endocervix. Diag Cytopathol 2000; 23 (1): 19-22.
- Carpenter AB et al: ThinPrep Pap Test: Performance and biopsy follow-up in a university hospital. Cancer Cytopathol 1999; 87 (3): 105-112.
- Hecht JL et al: Atypical glandular cells of undetermined significance in conventional cervical/vaginal smears and thin-layer preparations. Cancer 2002; Feb 25;96(1):1-4.
- Johnson JE and Rahemtulla A: Endocervical glandular neoplasia and its mimics in ThinPrep Pap Tests. A descriptive study. Acta Cytol 1999; 43(3): 369-75.
- Papillo J et al: Evaluation of the ThinPrep Pap Test in clinical practice: A seven-month case experience in northern Vermont. Acta Cytol 1999;42: 204-208.
- Selvaggi SM: Cytologic features of high-grade squamous intraepithelial lesions involving endocervical glands on ThinPrep® cytology. Diag Cytopathol 2002: 26, (3): 181-185.






















