Case Presentation
Case Presentation – June 2023
Clear Cell Sarcoma
Written by: Julissa Connelly, student, Cleveland Clinic School of Cytotechnology, Cleveland Ohio
Patient Age: 72-year-old male
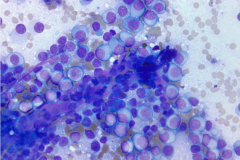
Specimen Type: 4R lymph node FNA, Diff Quik and Papanicolaou stained smears, ThinPrep® Non-Gyn, and Hematoxylin & Eosin-stained cell block
Patient History: Prior diagnosis of clear cell sarcoma in the left foot resected 20 years ago and was in surveillance
Cytologic Diagnosis: Malignant Neoplasm with melanocytic differentiation
Biopsy / Pathologic Diagnosis: Clear Cell Sarcoma
Case provided by: Cleveland Clinic
Clear Cell Sarcoma of the tendons and aponeuroses/soft tissues
Etiology:
Clear Cell Sarcoma (CCS) of soft tissues is a very rare tumor constituting less than 1% of all sarcomas.1,2 The genetic mutation involved in the development of CCS is a chromosomal translocation between chromosomes 22 and 12 that creates a Ewing sarcoma breakpoint region 1 (EWSR1)/activating transcription factor-1 (ATF1) fusion transcript, or rarely, between chromosomes 22 and 2 creating a EWSR1/CAMP responsive element binding protein 1 (CREB1) fusion transcript.3,4 EWSR1 is a protein that helps regulate transcription and mRNA splicing, and ATF1 and CREB1 are transcription factors that influence developmental processes.3,4 The result of the fusion transcript allows the tumor cells to avoid apoptosis and continue to proliferate.4 Although the exact etiology is unknown, other genetic and environmental factors are believed to play a role in the oncogenesis of CCS.3,5
Clinical Features:
Patients often present with a slowly enlarging mass in the extremities (foot and ankle) accompanied by some pain or tenderness, but in more advanced cases they may present with weight loss, malaise, and loss of appetite.1,2,6,7 In our case, the patient presented with 25-pound weight loss, reduced appetite, dysphagia, tachycardia, and profound fatigue/weakness. Computerized tomography (CT) imaging showed a large mediastinal mass, bulky lymphadenopathy, and a 15 mm left lower lobe nodule suspicious for lung cancer. However, a metastatic lesion could not be excluded. The FNA and biopsy from the 4R lymph node were positive for metastatic CCS. While most sarcomas tend to spread through a hematogenous route, CCS has a strong tendency to metastasize to regional and distant lymph nodes.2,6,7 Clear cell sarcomas are most often found in Caucasians, with no clear sex predilection, and are usually first noted in the 3rd-4th decade.5,7 The long gap between our patient’s first diagnosis and the present metastatic tumor is not uncommon. In one study, over 60% of patients that developed distant metastases had them occur 10 years after initial treatment.6
Treatment and Prognosis:
There are multiple treatment options for CCS. The preferred treatment is wide local excision of the tumor or amputation with negative margins; and if necessary, adjuvant radiation or chemotherapy.5,7 Chemotherapeutic regimens include cyclophosphamide, vincristine, dacarbazine, cisplatin, and doxorubicin, among others.5,6,7 Although rapidly fatal progression may occur, late metastases are quite common after many years of remission.6 Hence, the prognosis is poor, with a 5 year survival rate of about 50% and a 10 year survival rate of about 40%.5,6,7 The main prognostic factor for survival is tumor size.6,7
Cytology:
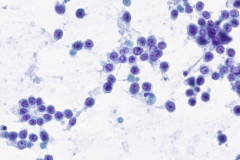
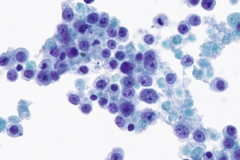
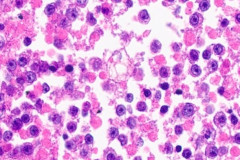
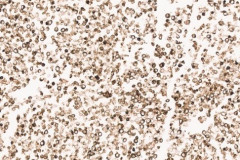
Clear cell sarcoma is moderately to markedly cellular with a clean or tigroid background.8 The tumor population is composed of round, polygonal, or spindle shaped cells with prominent central nucleoli in an eccentrically placed nucleus.8 The nuclei are slightly hyperchromatic with vesicular to coarse chromatin.8 Occasionally intranuclear cytoplasmic pseudoinclusions (INCIs) and wreathlike multinucleated giant cells can be seen. 8 There is a moderate amount of clear cytoplasm which can be finely vacuolated. Immunohistochemical (IHC) stains show tumor positivity for Melan-A, S-100, and HMB45.2,8 Our case showed a monotonous population of singly dispersed to loosely cohesive clusters of tumors cells with round to oval, hyperchromatic, eccentrically placed nuclei, coarse chromatin, prominent nucleoli, and moderate finely vacuolated cytoplasm. Wreathlike multinucleated giant cells were scant and INCIs were not present. IHC stains performed on the cell block showed tumor cells were positive for HMB45, SOX10, and BCL2 (focally). While the prior history of CCS was noted, based on cytomorphology and even with IHC stains, it was extremely difficult to differentiate melanoma from clear cell sarcoma. Hence the specimen was sent for fluorescence in situ hybridization (FISH). The result was positive for EWSR1 gene rearrangement, which is diagnostic for CCS.
Differential Diagnosis:
The differential diagnoses of CCS include malignant melanoma, PEComa and synovial sarcoma. A definitive diagnosis of CCS is extremely difficult on cytology, even with IHC stains, as evidenced by our case for which a definitive classification of clear cell sarcoma was deferred until FISH testing could be performed.
Malignant Melanoma (MM) is an aggressive cancer of the skin with high rates of metastases. The incidence of melanoma has significantly increased worldwide over the last 40 years, especially in young men and women.9 The cytology of MM consists of singly dispersed epithelioid, spindle, or pleomorphic tumor cells often with eccentrically placed nuclei.8 Binucleation, INCIs, prominent nucleoli, melanin pigment, and cytoplasmic vacuoles can be present.8 IHC stains that show tumor positivity for MM include SOX10, S-100, Melan-A, and HMB45.8,9 Due to similarities in cytology and immunohistochemical staining pattern of MM with CCS, ancillary testing (FISH) for the presence of EWSR1 gene rearrangement is helpful for a definitive diagnosis since it is present in CCS and absent in MM.2
PEComa of the lung, also known as the clear cell “sugar tumor” due to the presence of glycogen in the cytoplasm of the tumor cells, is a very rare, mostly benign tumor and can occur in any age group.8 The cells are bland, polygonal to spindle shaped with oval nuclei and clear cytoplasm.8 IHC stains often show tumor positivity for HMB45 and Melan-A and are negative for cytokeratins and CEA.8 Other IHC stains which are reportedly positive in PEComa include lysosomal markers such as cathepsin K and CD68.10 Although our case stained positive for HMB45, it was negative for Melan-A, and the positive FISH rearrangement for EWSR1 was helpful in distinguishing our case from a PEcoma.
Synovial sarcoma (SS) is a rare soft tissue tumor defined by the SYT-SSX fusion gene.11 It comprises about 10% of all sarcomas and often arises in the lower extremities of young adults.8 The cytology is highly cellular and composed of cohesive clusters of round, oval, or spindle cells frequently found in a pericapillary formation.11 When a glandular or epithelial component is present, which is best appreciated on histologic sections, they are subtyped as biphasic SS.11 The poorly differentiated type appears as singly dispersed, uniform small round blue cells with high nucleus-to-cytoplasmic ratios, delicate cytoplasm, and bland, oval nuclei that can have prominent nucleoli.8 IHCs stains will show tumor positivity for vimentin, epithelial membrane antigen (EMA), CD99, and BCL2. About 30% stain for S-100.8,11 Other studies have also shown tumor positivity for TLE1.11 In biphasic patterns, pan-cytokeratins and CK7 can also be positive.11 Recently, SSX antibody showed a sensitivity of 95% and specificity of 100% for SS. 12 Although our case showed focal positivity for BCL2, the tumor cells were negative for EMA and S-100.
References
- Juel J,Ibrahim RM. A case of clear cell sarcoma—A rare malignancy. International journal of surgery case reports. 2017;36:151-154. doi:https://doi.org/10.1016/j.ijscr.2017.05.034.
- Rodríguez-Martín M, Sáez-Rodríguez M, Esquivel B, Gonzáalez RS, Cabrera AN, Herrera AM. Clear cell sarcoma: a case mimicking primary cutaneous malignant melanoma. Indian J Dermatol. 2009;54(2):168-172. doi:10.4103/0019-5154.53193
- Wang WL, Mayordomo E, Zhang W, Hernandez VS, Tuvin D, Garcia L, Lev DC, Lazar AJ, Lopez-Terrada D. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts). Modern Pathology. 2009;22(9):1201-1209. https://doi.org/10.1038/modpathol.2009.85
- Cantile M, Marra L, Franco R, et al. Molecular detection and targeting of EWSR1 fusion transcripts in soft tissue tumors. Med Oncol. 2013;30(1):412. doi: 10.1007/s12032-012-0412-8
- Zamora EA, Cassaro S. Soft tissue clear cell sarcoma. StatPearls [Internet]. 2022. Available from:https://www.ncbi.nlm.nih.gov/books/NBK538426/
- Hocar O, Le Cesne A, Berissi S, et al. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012:984096. doi:10.1155/2012/984096
- Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 30 cases. Cancer: Interdisciplinary International Journal of the American Cancer Society. 1999;86(6):969-975. https://doi.org/10.1002/(SICI)1097-0142(19990915)86:6<969::AID-CNCR11>3.0.CO;2-Z
- Cibas ES, Ducatman BS. Cytology: Diagnostic Principles and Clinical Correlates. 5th ed. Elsevier Inc; 2021.
- Ronchi A, Montella M, Zito Marino F, Argenziano G, Moscarella E, Brancaccio G, Ferraro G, Nicoletti GF, Troiani T, Franco R, Cozzolino I. Cytologic diagnosis of metastatic melanoma by FNA: A practical review. Cancer Cytopathology. 2022;130(1):18-29.
- Caliò A, Mengoli MC, Cavazza A, Rossi G, Ghimenton C, Brunelli M, Pea M, Chilosi M, Marcolini L, Martignoni G. Cathepsin K expression in clear cell “sugar” tumor (PEComa) of the lung. Virchows Arch. 2018 Jul;473(1):55-59.
- Zhang Y, Wessman S, Wejde J, Tani E, Haglund F. Diagnosing synovial sarcoma by fine-needle aspiration cytology and molecular techniques. Cytopathology. 2019 Sep;30(5):504-509
- Baranov E, McBride MJ, Bellizzi AM, Ligon AH, Fletcher CDM, Kadoch C, Hornick JL. A Novel SS18-SSX Fusion-specific Antibody for the Diagnosis of Synovial Sarcoma. Am J Surg Pathol. 2020 Jul;44(7):922-933.