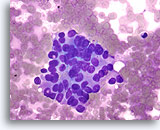
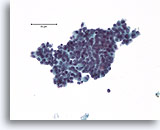
Note: The cell block images presented here were generated using a manual method during development of the automated instrument.
FNA of the breast is an effective means of identifying breast cancers to allow definitive treatment, while accurately excluding malignancy in most benign lesions. [1, 2] Compared to core biopsy, there is decreased morbidity, including hematoma, infection, pain, and risk of seeding of the biopsy track. Nevertheless, the past decade has seen a decline in breast FNA in favor of more aggressive core biopsy techniques. Some pathologists prefer the histologic evaluation of core biopsies because they can be analyzed relatively quickly and easily, and they allow immunohistochemistry (IHC) to be applied. Cell blocks of breast FNA’s offer these same advantages. Combining FNA with core biopsies has been shown to increase diagnostic accuracy. [3] Our recent experience suggests that combining a cytology preparation of a breast FNA with a cell block can also combine the advantages of both approaches. [4]
The major problem in classifying proliferative ductal lesions by cytology is that tissue-level changes define the degree of hyperplasia rather than cytologic changes. [7] Hyperplasia is defined as the stratification of ductal cells away from the basement membrane microenvironment. The degree of hyperplasia is graded histologically into usual ductal hyperplasia and atypical ductal hyperplasia.
The major problem in classifying proliferative ductal lesions by cytology is that tissue-level changes define the degree of hyperplasia rather than cytologic changes. [7] Hyperplasia is defined as the stratification of ductal cells away from the basement membrane microenvironment. The degree of hyperplasia is graded histologically into usual ductal hyperplasia and atypical ductal hyperplasia.
In usual ductal hyperplasia, polarity between adjacent cells is often focally maintained with alignment of the long axis of cells in the same direction. This alignment gives a streaming, or vague « school of fish », appearance to the stratified population. The long axis of the cells in usual hyperplasia also tends to align itself with any residual lumina within the duct, and the residual lumina tend to have an elongated shape with a fuzzy edge. Nuclear spacing in usual hyperplasia tends to vary over the diameter of the duct. Usual hyperplasia often shows an admixture of a distinctly different myoepithelial cell population admixed with the ductal cells. Note that pagetoid extension of mammary carcinoma in benign ducts can simulate an admixture of cell types. [7] Finally, in usual hyperplasia, the overall appearance of the cells may vary predictably depending on how far the cells have stratified away from the basement membrane. Commonly, the nucleus becomes darker and cytoplasm becomes denser as the cells stratify further from the native basement membrane zone.[8]
Atypical ductal hyperplasia is characterized by a stratifying population with loss of shared polarity (no streaming) between adjacent cells, no apparent admixed myoepithelial cells, residual lumina that become more smoothly rounded (« punched out ») with the long axis of the nuclei randomized with respect to the edges of the lumina, and no apparent maturation of the cells as they stratify further from the basement membrane zone. [8] In most cases, these features are easy to note at low magnification in histologic sections. Ductal carcinoma in situ is diagnosed when the above features of atypical hyperplasia are unequivocally developed and the population of cells extends over many ducts.
Many of these diagnostic histologic features that distinguish the degree of hyperplasia are difficult or impossible to discern in cytology preparations unless high magnification is used to focus up and down through piles of cells. On the other hand, there are some features that cytologists can use to characterize and recognize ductal proliferations that surgical pathologists cannot use. Discohesion of the ductal cells is one important feature. Cell blocks capture individual discohesive cells to allow this important feature to be noted.
It is important to note that nuclear atypia is not characteristic of low grade ductal carcinoma in situ or atypical hyperplasia. Nuclear atypia is a feature of high grade ductal carcinomas. Cell blocks preserve nuclear features crisply, comparable to cytology preparations, allowing both the low grade architectural changes and the high grade cytologic changes to be detected.
A difficult differential diagnosis in breast FNA is the distinction between fibroadenoma, papilloma and papillary carcinoma. The distinction is important because fibroadenomas, if confidently diagnosed, do not have to be excised. Central papillomas do not necessarily need to be excised, or require only gross excision. On the other hand, papillary carcinomas require careful complete excision with clear margins. The distinction between papilloma and papillary carcinoma is defined by the absence of a myoepithelial cell population in the latter, or the presence of stratification that meets architectural criteria for in-situ carcinoma. Myoepithelial cells can be difficult or impossible to discern in monolayer cytology preparations and papillomas can show considerable stratification and nuclear atypia. Thus the distinction of papillary carcinoma and papilloma is widely considered to be nearly impossible on cytology preparations alone. While some residual myoepithelial cells are often retained next to the basement membrane in ductal carcinoma in situ, a non-invasive papillary carcinoma infrequently shows residual myoepithelial cells. [7] Immunohistochemical staining for myoepithelial cells (calponin, p63, and smooth muscle actin) in cell block sections can be very useful to distinguish papillomas from papillary carcinomas. [9, 10, 11, 12]
Distinction of lobular from ductal carcinoma is difficult by cytopathology. The distinction can occasionally be important. Since lobular carcinoma in situ is sometimes not treated surgically, the rare occurrence of lobular carcinoma in situ as an incidental finding (e.g., colonizing a fibroadenoma) could lead to excessive surgery. On FNA cytology preparations, lobular carcinomas are generally very sparsely cellular and are easily under-diagnosed. Not only are there generally few cells, the cells can be very bland. Lack of polarity in the sparse cells, and the presence of « targetoid » mucin vacuoles is a helpful diagnostic trait of lobular carcinoma. Intracellular mucin is very rare in normal ductal cells, and its presence can be demonstrated in cell blocks. Lobular carcinoma is characterized by loss of E-Cadherin expression which can be detected in histologic sections by immunohistochemistry [12]. We have noted that the stromal tissue fragments obtained by FNA often have entrapped lobular carcinoma cells that can be seen in cell blocks, suggesting that the addition of a cell block to breast FNA can help improve the detection and specific diagnosis of lobular carcinoma.
Cytology preparations do not allow the presence of invasion to be diagnosed. [13] Since in situ cancers may not need lymph node sampling, this has been an important limitation of breast FNA for planning treatment. Through the use of cell blocks, we recently found that invasion can be diagnosed in a significant proportion of breast cancer FNA’s. [4]
In this chapter, we will show that combining cytology preparations with cell blocks allows the best of both worlds: micro-sized biopsies obtained by FNA allow cytologic as well as histologic criteria to be applied.
Reminder: You may click on any slide image
for an enlarged view.
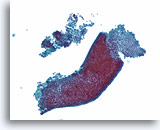
Figure 1
Benign ductal cells, Breast FNA, ThinPrep®.
Normal ductal cells form a single cell layer attached to a basement membrane. The 2-dimensionally arranged ductal cells are cohesive with each other but strip away with ease from the basement membrane in fine needle aspirates. When seen in a monolayer preparation, the ductal cells typically lie as a flat sheet parallel to the slide surface (right). They can also remain as an intact single cell thick duct (lower portion). Smaller ductules (upper left) can show more complex shapes and it may be more difficult to appreciate that the cells are still only one cell layer thick without focusing up and down.20X
Figure 1
Benign ductal cells, Breast FNA, ThinPrep®.
Normal ductal cells form a single cell layer attached to a basement membrane. The 2-dimensionally arranged ductal cells are cohesive with each other but strip away with ease from the basement membrane in fine needle aspirates. When seen in a monolayer preparation, the ductal cells typically lie as a flat sheet parallel to the slide surface (right). They can also remain as an intact single cell thick duct (lower portion). Smaller ductules (upper left) can show more complex shapes and it may be more difficult to appreciate that the cells are still only one cell layer thick without focusing up and down.20X
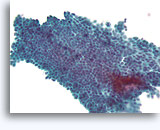
Figure 2A
Benign ductal cells, Breast FNA, ThinPrep®.
Myoepithelial cells are another cell type that normally lie between the basement membrane and the ductal cells. They typically have slightly darker nuclei, and paler fragile cytoplasm that may rupture when the ductal cells strip from the basement membrane.
40X
Figure 2A
Benign ductal cells, Breast FNA, ThinPrep®.
Myoepithelial cells are another cell type that normally lie between the basement membrane and the ductal cells. They typically have slightly darker nuclei, and paler fragile cytoplasm that may rupture when the ductal cells strip from the basement membrane.
40X
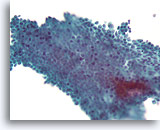
Figure 2B
Benign ductal cells, Breast FNA, ThinPrep®.
By focusing up and down, one can see that the myoepithelial cells of a normal duct lie loosely but predictably in a plane parallel to the ductal cells.
40X
Figure 2B
Benign ductal cells, Breast FNA, ThinPrep®.
By focusing up and down, one can see that the myoepithelial cells of a normal duct lie loosely but predictably in a plane parallel to the ductal cells.
40X
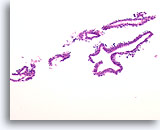
Figure 3
Benign ductal cells, Breast FNA, Cell Block.
What appeared as a flat sheet on ThinPrep® appears as a line of cells. It is easy to see at low magnification that benign ductal cells are only one cell in thickness. Note the loosely adherent myoepithelial cells around the outside of the duct.
20X
Figure 3
Benign ductal cells, Breast FNA, Cell Block.
What appeared as a flat sheet on ThinPrep® appears as a line of cells. It is easy to see at low magnification that benign ductal cells are only one cell in thickness. Note the loosely adherent myoepithelial cells around the outside of the duct.
20X
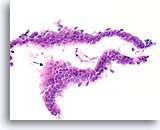
Figure 4
Benign ductal cells, Breast FNA, Cell Block.
One can tell that the cells are not truly piled up away from the basement membrane-myoepithelial zone by noting that the cytoplasm becomes predictably more abundant at the edge of the tangential area (arrow).
40X
Figure 4
Benign ductal cells, Breast FNA, Cell Block.
One can tell that the cells are not truly piled up away from the basement membrane-myoepithelial zone by noting that the cytoplasm becomes predictably more abundant at the edge of the tangential area (arrow).
40X
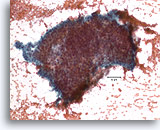
Figure 5
Proliferative ductal lesion, Breast FNA, Direct smear.
Stratification of the ductal cells is known as hyperplasia or proliferative change. Obscuring blood and the thickness of the fragments make it difficult to gauge how much hyperplasia is present.
20X
Figure 5
Proliferative ductal lesion, Breast FNA, Direct smear.
Stratification of the ductal cells is known as hyperplasia or proliferative change. Obscuring blood and the thickness of the fragments make it difficult to gauge how much hyperplasia is present.
20X
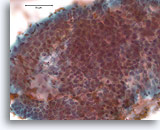
Figure 6
Proliferative ductal lesion, Breast FNA, Direct smear.
At higher magnification, by focusing up and down, one can appreciate some probable myoepithelial cells admixed with the ductal cells, and probable slit-like spaces. These two features are characteristic of usual-type ductal hyperplasia, a lesion that conveys a roughly two fold increased relative risk of subsequent breast cancer [14].
40X
Figure 6
Proliferative ductal lesion, Breast FNA, Direct smear.
At higher magnification, by focusing up and down, one can appreciate some probable myoepithelial cells admixed with the ductal cells, and probable slit-like spaces. These two features are characteristic of usual-type ductal hyperplasia, a lesion that conveys a roughly two fold increased relative risk of subsequent breast cancer [14].
40X
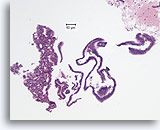
Figure 7
Usual ductal hyperplasia, Breast FNA, Cell Block.
This needle rinse from the case in Figure 5 shows benign ductal cells as single-cell thick strips of epithelium on the right half of the image. On the left is an area of usual hyperplasia. The stratification of the cells in this area cannot be explained by tangential sectioning. From this low magnification, one can see slit-like spaces interrupting the stratifying ductal cells. Also characteristic of usual ductal hyperplasia is the presence of an admixture of cells with differing cytologic features (myoepithelial cells) in the stratifying population.
10X
Figure 7
Usual ductal hyperplasia, Breast FNA, Cell Block.
This needle rinse from the case in Figure 5 shows benign ductal cells as single-cell thick strips of epithelium on the right half of the image. On the left is an area of usual hyperplasia. The stratification of the cells in this area cannot be explained by tangential sectioning. From this low magnification, one can see slit-like spaces interrupting the stratifying ductal cells. Also characteristic of usual ductal hyperplasia is the presence of an admixture of cells with differing cytologic features (myoepithelial cells) in the stratifying population.
10X
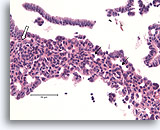
Figure 8
Usual ductal hyperplasia, Breast FNA, Cell Block.
Note the admixture of darker and lighter-staining nuclei, and the elongated spaces. In usual ductal hyperplasia, the cells tend to align along the axis of the spaces (arrows). Another useful diagnostic trait is the alignment of the long axis of the nuclei of adjacent cells, with a streaming or vague « school of fish » pattern (open arrow). These features, evident at relatively low magnification in histologic sections, can be difficult or impossible to appreciate in cytology preparations.
40X
Figure 8
Usual ductal hyperplasia, Breast FNA, Cell Block.
Note the admixture of darker and lighter-staining nuclei, and the elongated spaces. In usual ductal hyperplasia, the cells tend to align along the axis of the spaces (arrows). Another useful diagnostic trait is the alignment of the long axis of the nuclei of adjacent cells, with a streaming or vague « school of fish » pattern (open arrow). These features, evident at relatively low magnification in histologic sections, can be difficult or impossible to appreciate in cytology preparations.
40X
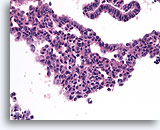
Figure 9
Usual ductal hyperplasia, Breast FNA, Cell Block.
Benign ductal cells above with mild columnar change contrast with the area of usual ductal hyperplasia below. Note the admixture of distinctly different cell types in the center, some with large pale nuclei and others with small dark nuclei.
40X
Figure 9
Usual ductal hyperplasia, Breast FNA, Cell Block.
Benign ductal cells above with mild columnar change contrast with the area of usual ductal hyperplasia below. Note the admixture of distinctly different cell types in the center, some with large pale nuclei and others with small dark nuclei.
40X
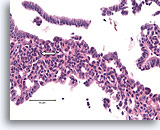
Figure 10
Usual ductal hyperplasia, Breast FNA, Cell Block.
Note the streaming of nuclei in the area to the left, reminiscent of a « school of fish » (arrow).
40X
Figure 10
Usual ductal hyperplasia, Breast FNA, Cell Block.
Note the streaming of nuclei in the area to the left, reminiscent of a « school of fish » (arrow).
40X
Figure 11
Atypical ductal proliferative lesion, Breast FNA, Diff-Quick stained smear.
Ductal cells are piled up in three dimensions. There is no distinct admixed population of myoepithelial cells, and there is only focal possible shared polarity of adjacent ductal cells in the lower left.
60X
Figure 11
Atypical ductal proliferative lesion, Breast FNA, Diff-Quick stained smear.
Ductal cells are piled up in three dimensions. There is no distinct admixed population of myoepithelial cells, and there is only focal possible shared polarity of adjacent ductal cells in the lower left.
60X
Figure 12
Atypical ductal proliferative lesion, Breast FNA, ThinPrep®.
Note the marked stratification of cells. A vague streaming pattern is evident in the center favoring a benign ductal hyperplasia, but without being able to characterize the architectural arrangement of the cells, it is not possible to definitively exclude atypical ductal hyperplasia.
40X
Figure 12
Atypical ductal proliferative lesion, Breast FNA, ThinPrep®.
Note the marked stratification of cells. A vague streaming pattern is evident in the center favoring a benign ductal hyperplasia, but without being able to characterize the architectural arrangement of the cells, it is not possible to definitively exclude atypical ductal hyperplasia.
40X
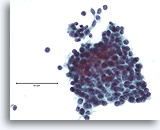
Figure 13
Atypical ductal proliferative lesion, Breast FNA, ThinPrep®.
Atypical features include the slightly discohesive ductal population (note the individual ductal cells), the presence of a stratifying population of ductal cells without an obvious admixture of myoepithelial cells, and the near absence of a shared polarity among the ductal cells.
60X
Figure 13
Atypical ductal proliferative lesion, Breast FNA, ThinPrep®.
Atypical features include the slightly discohesive ductal population (note the individual ductal cells), the presence of a stratifying population of ductal cells without an obvious admixture of myoepithelial cells, and the near absence of a shared polarity among the ductal cells.
60X
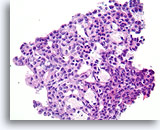
Figure 14
Usual ductal hyperplasia, Breast FNA, Cell Block.
Note how the cytoplasmic features change predictably toward the lower right of the field. This feature of usual hyperplasia is difficult to appreciate in cytology preparations. There is also some alignment of the long axis of the ductal cell nuclei with a subtle but distinctive benign streaming pattern, especially toward the lower right. Note also the alignment of ductal cell nuclei parallel with the elongated spaces.
40X
Figure 14
Usual ductal hyperplasia, Breast FNA, Cell Block.
Note how the cytoplasmic features change predictably toward the lower right of the field. This feature of usual hyperplasia is difficult to appreciate in cytology preparations. There is also some alignment of the long axis of the ductal cell nuclei with a subtle but distinctive benign streaming pattern, especially toward the lower right. Note also the alignment of ductal cell nuclei parallel with the elongated spaces.
40X
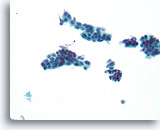
Figure 15
Suspicious ductal proliferative lesion, Breast FNA, ThinPrep®.
Except for some possible shared polarity in the ductal cell group just left of center, the findings are worrisome due to the presence of a monomorphic population of cells that stratify and show discohesion.
40X
Figure 15
Suspicious ductal proliferative lesion, Breast FNA, ThinPrep®.
Except for some possible shared polarity in the ductal cell group just left of center, the findings are worrisome due to the presence of a monomorphic population of cells that stratify and show discohesion.
40X
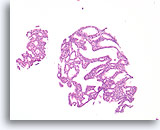
Figure 16
Atypical ductal hyperplasia, Breast FNA, Cell Block.
This image from the needle rinse of the case in Figure 15 shows a uniform population of freely stratifying ductal cells with uniform spacing of nuclei, absence of a streaming pattern, and the presence of more uniformly rounded spaces in the smaller fragment.
10X
Figure 16
Atypical ductal hyperplasia, Breast FNA, Cell Block.
This image from the needle rinse of the case in Figure 15 shows a uniform population of freely stratifying ductal cells with uniform spacing of nuclei, absence of a streaming pattern, and the presence of more uniformly rounded spaces in the smaller fragment.
10X
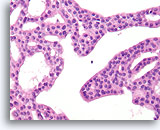
Figure 17
Atypical ductal hyperplasia, Breast FNA, Cell Block.
Note the uniformity of the cells (indicating an absence of myoepithelial cells), and the uniform spacing of cells without shared polarity.
40X
Figure 17
Atypical ductal hyperplasia, Breast FNA, Cell Block.
Note the uniformity of the cells (indicating an absence of myoepithelial cells), and the uniform spacing of cells without shared polarity.
40X
Figure 18
Ductal proliferative lesion, Papillary neoplasm vs. fibroadenoma, Breast FNA, Direct smear.
Blood partially obscures cellular detail, but a large cluster of proliferative ductal cells can be seen on the left. A stromal fragment with rounded edges is seen on the right.
10X
Figure 18
Ductal proliferative lesion, Papillary neoplasm vs. fibroadenoma, Breast FNA, Direct smear.
Blood partially obscures cellular detail, but a large cluster of proliferative ductal cells can be seen on the left. A stromal fragment with rounded edges is seen on the right.
10X
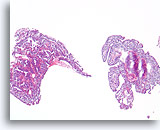
Figure 19
Papilloma, Breast FNA, Cell Block.
This image from the same FNA in Figure 18 shows an obvious papillary architecture.
10X
Figure 19
Papilloma, Breast FNA, Cell Block.
This image from the same FNA in Figure 18 shows an obvious papillary architecture.
10X
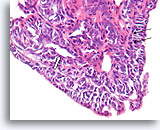
Figure 20
Papilloma, Breast FNA, Cell Block.
Higher magnification shows a mixed cell population lining the surface and within the underlying ducts. A row of myoepithelial cells is evident between the ductal cells and the underlying stroma (arrow). Note the slit-like space characteristic of usual hyperplasia (open arrow).
40X
Figure 20
Papilloma, Breast FNA, Cell Block.
Higher magnification shows a mixed cell population lining the surface and within the underlying ducts. A row of myoepithelial cells is evident between the ductal cells and the underlying stroma (arrow). Note the slit-like space characteristic of usual hyperplasia (open arrow).
40X
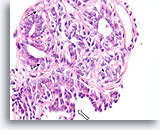
Figure 21
Papilloma, Breast FNA, Cell Block.
Note the admixture of pale myoepithelial cells (arrows) and the maturation of the ductal cells as they stratify further from the basement membrane (open arrow).
40X
Figure 21
Papilloma, Breast FNA, Cell Block.
Note the admixture of pale myoepithelial cells (arrows) and the maturation of the ductal cells as they stratify further from the basement membrane (open arrow).
40X

Figure 22
Papilloma, Breast FNA, ThinPrep®.
When fibrovascular cores are stripped of ductal cells, the papillary nature of the lesion is readily apparent. Note the smooth surface of the stromal cores, indicating that the stromal collagen was laid down in cooperation with an overlying epithelial population. If the stromal surfaces are rough, it may not be possible in a cytology preparation to definitively diagnose a papillary neoplasm.
40X
Figure 22
Papilloma, Breast FNA, ThinPrep®.
When fibrovascular cores are stripped of ductal cells, the papillary nature of the lesion is readily apparent. Note the smooth surface of the stromal cores, indicating that the stromal collagen was laid down in cooperation with an overlying epithelial population. If the stromal surfaces are rough, it may not be possible in a cytology preparation to definitively diagnose a papillary neoplasm.
40X
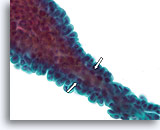
Figure 23
Papilloma, Breast FNA, ThinPrep®.
When the papillae are thin, fibrovascular cores can be identified in cytology preparations. When the overlying ductal cells are not too stratified, myoepithelial cells can be tentatively identified underlying the ductal cells (open arrows) to favor the diagnosis of papilloma over papillary carcinoma.
60X
Figure 23
Papilloma, Breast FNA, ThinPrep®.
When the papillae are thin, fibrovascular cores can be identified in cytology preparations. When the overlying ductal cells are not too stratified, myoepithelial cells can be tentatively identified underlying the ductal cells (open arrows) to favor the diagnosis of papilloma over papillary carcinoma.
60X
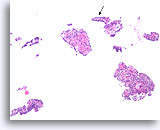
Figure 24
Papilloma, Breast FNA, Cell Block.
This image from the case in Figure 23 shows obvious papillary architecture. The thin papillary frond at the arrow is roughly the same size as the frond shown in the previous figure.
10X
Figure 24
Papilloma, Breast FNA, Cell Block.
This image from the case in Figure 23 shows obvious papillary architecture. The thin papillary frond at the arrow is roughly the same size as the frond shown in the previous figure.
10X
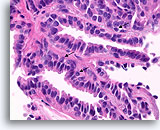
Figure 25
Papilloma, Breast FNA, Cell Block.
Histologic sectioning allows study of the composition, architecture, and cytologic features of the larger papillary fragments. Myoepithelial cells are readily present (arrows), and no stratification of the ductal cells is seen.
60X
Figure 25
Papilloma, Breast FNA, Cell Block.
Histologic sectioning allows study of the composition, architecture, and cytologic features of the larger papillary fragments. Myoepithelial cells are readily present (arrows), and no stratification of the ductal cells is seen.
60X
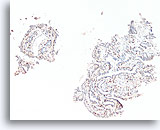
Figure 26
Papilloma, Breast FNA, Cell Block.
A p63 immunostain in the case shown in Figures 23-25 helps to highlight the myoepithelial cells that are sprinkled throughout this papilloma.
10X
Figure 26
Papilloma, Breast FNA, Cell Block.
A p63 immunostain in the case shown in Figures 23-25 helps to highlight the myoepithelial cells that are sprinkled throughout this papilloma.
10X
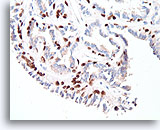
Figure 27
Papilloma, Breast FNA, Cell Block.
The myoepithelial cells form almost continuous rows underlying the ductal cells (p63 immunostain).
40X
Figure 27
Papilloma, Breast FNA, Cell Block.
The myoepithelial cells form almost continuous rows underlying the ductal cells (p63 immunostain).
40X
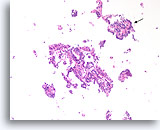
Figure 28
Papilloma, Breast FNA, Cell Block.
Discohesive single cells are present in the background that can be a worrisome feature of papillary neoplasms. A feature at this low magnification that favors papilloma over papillary carcinoma is the differing appearance of the ductal cells in different areas. Note the compact dark ductal cells in the upper fragment (arrow) compared to the slightly paler ductal cells in the larger fragment. Papillary carcinomas tend to be much more uniform.
10X
Figure 28
Papilloma, Breast FNA, Cell Block.
Discohesive single cells are present in the background that can be a worrisome feature of papillary neoplasms. A feature at this low magnification that favors papilloma over papillary carcinoma is the differing appearance of the ductal cells in different areas. Note the compact dark ductal cells in the upper fragment (arrow) compared to the slightly paler ductal cells in the larger fragment. Papillary carcinomas tend to be much more uniform.
10X
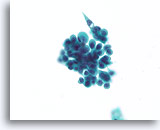
Figure 29
Proliferative change in papilloma, Breast FNA, ThinPrep®.
In addition to discohesion, papillomas commonly show a worrisome stratification. A clue to the diagnosis of papilloma in this case is the waxy squamous-appearing cytoplasm (not like the granular apocrine metaplastic cytoplasm).
60X
Figure 29
Proliferative change in papilloma, Breast FNA, ThinPrep®.
In addition to discohesion, papillomas commonly show a worrisome stratification. A clue to the diagnosis of papilloma in this case is the waxy squamous-appearing cytoplasm (not like the granular apocrine metaplastic cytoplasm).
60X
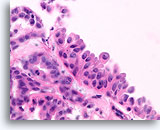
Figure 30
Papilloma, Breast FNA, Cell Block.
A histologic section of a larger fragment shows the origin of the stratifying ductal cells with squamoid change.
60X
Figure 30
Papilloma, Breast FNA, Cell Block.
A histologic section of a larger fragment shows the origin of the stratifying ductal cells with squamoid change.
60X
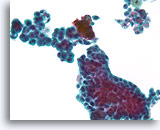
Figure 31
Proliferative change in papilloma, Breast FNA, ThinPrep®.
The stratification, loss of polarity and slight nuclear atypia can be prominent in some papillomas. The key to the diagnosis is to be able to see the large scale architecture of the fragments, but this is difficult in cytology preparations.
40X
Figure 31
Proliferative change in papilloma, Breast FNA, ThinPrep®.
The stratification, loss of polarity and slight nuclear atypia can be prominent in some papillomas. The key to the diagnosis is to be able to see the large scale architecture of the fragments, but this is difficult in cytology preparations.
40X
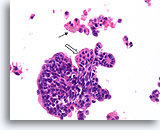
Figure 32
Papilloma, Breast FNA, Cell Block.
A histologic section of the FNA shown in Figure 31 shows a comparable fragment with discohesive cells in the background. The presence of hemosiderin in macrophages (arrow) is an important clue to the papillary nature of this lesion. Note the benign streaming pattern of the ductal cells. Focal squamoid change is present (open arrow).
40X
Figure 32
Papilloma, Breast FNA, Cell Block.
A histologic section of the FNA shown in Figure 31 shows a comparable fragment with discohesive cells in the background. The presence of hemosiderin in macrophages (arrow) is an important clue to the papillary nature of this lesion. Note the benign streaming pattern of the ductal cells. Focal squamoid change is present (open arrow).
40X
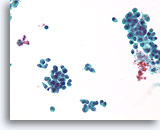
Figure 33
Proliferative change in a papilloma, Breast FNA, ThinPrep®.
The discohesion of the ductal cells in a papilloma can be alarming.
40X
Figure 33
Proliferative change in a papilloma, Breast FNA, ThinPrep®.
The discohesion of the ductal cells in a papilloma can be alarming.
40X
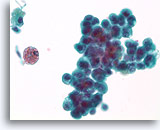
Figure 34
Proliferative change and cytologic atypia in papilloma, Breast FNA, ThinPrep®.
Nuclear atypia can become quite marked in papillomas, particularly in the areas with squamoid change. Nuclear atypia by itself is not a reliable means of distinguishing papillomas from papillary carcinomas. An important clue to the papillary nature of this proliferation is the hemosiderin and the squamoid cytoplasm.
60X
Figure 34
Proliferative change and cytologic atypia in papilloma, Breast FNA, ThinPrep®.
Nuclear atypia can become quite marked in papillomas, particularly in the areas with squamoid change. Nuclear atypia by itself is not a reliable means of distinguishing papillomas from papillary carcinomas. An important clue to the papillary nature of this proliferation is the hemosiderin and the squamoid cytoplasm.
60X
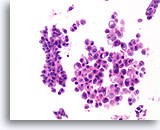
Figure 35
Papilloma, Breast FNA, Cell Block.
The discohesive and cytologically atypical nature of the population seen in Figure 34 can be appreciated in the corresponding cell block section. The variability between the two largest groups of ductal cells favors a benign diagnosis.
40X
Figure 35
Papilloma, Breast FNA, Cell Block.
The discohesive and cytologically atypical nature of the population seen in Figure 34 can be appreciated in the corresponding cell block section. The variability between the two largest groups of ductal cells favors a benign diagnosis.
40X
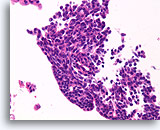
Figure 36
Papilloma, Breast FNA, Cell Block.
The streaming pattern evident in other areas of the FNA shown in Figure 35 is important features strongly favoring papilloma over papillary carcinoma.
40X
Figure 36
Papilloma, Breast FNA, Cell Block.
The streaming pattern evident in other areas of the FNA shown in Figure 35 is important features strongly favoring papilloma over papillary carcinoma.
40X
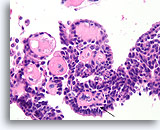
Figure 37
Papilloma, Breast FNA, Cell Block.
Fibrovascular cores are easy to see in this region of the cell block from the same case shown in Figures 33-36. Note the line of myoepithelial cells (arrow).
40X
Figure 37
Papilloma, Breast FNA, Cell Block.
Fibrovascular cores are easy to see in this region of the cell block from the same case shown in Figures 33-36. Note the line of myoepithelial cells (arrow).
40X
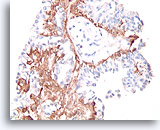
Figure 38
Papilloma, Breast FNA, Cell Block.
A calponin immunostain makes it easier to see the myoepithelial cell population that underlies the ductal cells.
40X
Figure 38
Papilloma, Breast FNA, Cell Block.
A calponin immunostain makes it easier to see the myoepithelial cell population that underlies the ductal cells.
40X
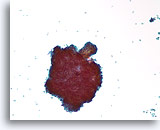
Figure 39
Papillary neoplasm, Breast FNA, ThinPrep®.
Scattered individual ductal cells and a large cluster with a fibrovascular stalk are seen.
10X
Figure 39
Papillary neoplasm, Breast FNA, ThinPrep®.
Scattered individual ductal cells and a large cluster with a fibrovascular stalk are seen.
10X
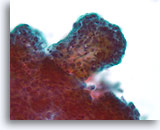
Figure 40
Papillary neoplasm, Breast FNA, ThinPrep®.
A higher magnification shows the fibrovascular stalk but it is difficult to study the architectural features of the ductal cells in the lower left of this thick fragment.
40X
Figure 40
Papillary neoplasm, Breast FNA, ThinPrep®.
A higher magnification shows the fibrovascular stalk but it is difficult to study the architectural features of the ductal cells in the lower left of this thick fragment.
40X
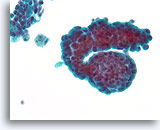
Figure 41
Papillary neoplasm, Breast FNA, ThinPrep®.
This group of ductal cells shows marked proliferative change with stratification. Two macrophages with trace hemosiderin are a clue to the presence of a papillary neoplasm.
40X
Figure 41
Papillary neoplasm, Breast FNA, ThinPrep®.
This group of ductal cells shows marked proliferative change with stratification. Two macrophages with trace hemosiderin are a clue to the presence of a papillary neoplasm.
40X
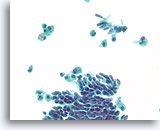
Figure 42
Papillary neoplasm, Breast FNA, ThinPrep®.
Columnar change and macrophages with hemosiderin are a clue to the diagnosis of papillary neoplasm. Columnar change can give the erroneous impression of a benign streaming pattern.
40X
Figure 42
Papillary neoplasm, Breast FNA, ThinPrep®.
Columnar change and macrophages with hemosiderin are a clue to the diagnosis of papillary neoplasm. Columnar change can give the erroneous impression of a benign streaming pattern.
40X
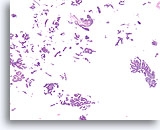
Figure 43
Papillary neoplasm, Breast FNA, Cell Block.
A low magnification of the residual needle rinse from the case in Figures 39-42 shows an obvious papillary architecture. Note the similarity of the ductal population throughout this field. This monotonous pattern is more suggestive of papillary carcinoma than papilloma.
40X
Figure 43
Papillary neoplasm, Breast FNA, Cell Block.
A low magnification of the residual needle rinse from the case in Figures 39-42 shows an obvious papillary architecture. Note the similarity of the ductal population throughout this field. This monotonous pattern is more suggestive of papillary carcinoma than papilloma.
40X
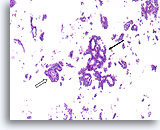
Figure 44
Papillary carcinoma, Breast FNA, Cell Block.
Higher magnification of the case shown in Figures 39-43 shows a fibrovascular core (open arrow) and an area of cribriforming consistent with papillary carcinoma (arrow).
10X
Figure 44
Papillary carcinoma, Breast FNA, Cell Block.
Higher magnification of the case shown in Figures 39-43 shows a fibrovascular core (open arrow) and an area of cribriforming consistent with papillary carcinoma (arrow).
10X
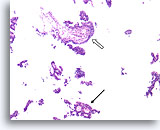
Figure 45
Papillary carcinoma, Breast FNA, Cell Block.
Another area of cribriforming (arrow) and a fibrovascular core (open arrow) are easily discerned.
10X
Figure 45
Papillary carcinoma, Breast FNA, Cell Block.
Another area of cribriforming (arrow) and a fibrovascular core (open arrow) are easily discerned.
10X
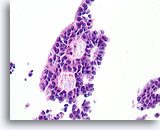
Figure 46
Papillary carcinoma, Breast FNA, Cell Block.
Higher magnification of the diagnostic cribriform pattern from Figure 45 shows a monotonous cell population with randomized polarity forming smooth, rounded spaces. Necrotic debris is focally present in one lumen.
40X
Figure 46
Papillary carcinoma, Breast FNA, Cell Block.
Higher magnification of the diagnostic cribriform pattern from Figure 45 shows a monotonous cell population with randomized polarity forming smooth, rounded spaces. Necrotic debris is focally present in one lumen.
40X
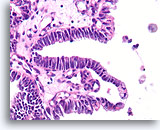
Figure 47
Papillary carcinoma, Breast FNA, Cell Block.
In areas, the papillary carcinoma shows no stratification with an absence of myoepithelial cells.
40X
Figure 47
Papillary carcinoma, Breast FNA, Cell Block.
In areas, the papillary carcinoma shows no stratification with an absence of myoepithelial cells.
40X
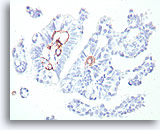
Figure 48
Papillary carcinoma, Breast FNA, Cell Block.
This smooth muscle actin stain shows some stromal myofibroblasts, but no myoepithelial cells beyond the basement membrane confines of the fibrovascular cores in the papillary carcinoma shown in Figures 39-47.
40X
Figure 48
Papillary carcinoma, Breast FNA, Cell Block.
This smooth muscle actin stain shows some stromal myofibroblasts, but no myoepithelial cells beyond the basement membrane confines of the fibrovascular cores in the papillary carcinoma shown in Figures 39-47.
40X
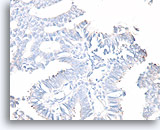
Figure 49
Papillary carcinoma, Breast FNA, Cell Block.
A calponin stain for myoepithelial cells is often easier to interpret than a smooth muscle actin immunostain. The same case as Figure 48 shows no myoepithelial cells.
40X
Figure 49
Papillary carcinoma, Breast FNA, Cell Block.
A calponin stain for myoepithelial cells is often easier to interpret than a smooth muscle actin immunostain. The same case as Figure 48 shows no myoepithelial cells.
40X
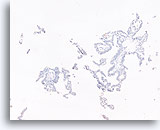
Figure 50
Papillary carcinoma, Breast FNA, Cell Block.
A p63 immunostain for myoepithelial cells is also negative in the case shown in the preceding figures, supporting the diagnosis of papillary carcinoma.
10X
Figure 50
Papillary carcinoma, Breast FNA, Cell Block.
A p63 immunostain for myoepithelial cells is also negative in the case shown in the preceding figures, supporting the diagnosis of papillary carcinoma.
10X
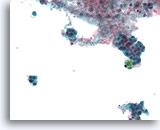
Figure 51
Papillary neoplasm, Breast FNA, ThinPrep®.
A different FNA from the previous series shows discohesive ductal cells with proliferative changes and hemosiderin.
40X
Figure 51
Papillary neoplasm, Breast FNA, ThinPrep®.
A different FNA from the previous series shows discohesive ductal cells with proliferative changes and hemosiderin.
40X
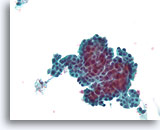
Figure 52
Papillary neoplasm, Breast FNA, ThinPrep®.
Marked proliferative changes are present. A fibrovascular core is difficult to discern.
40X
Figure 52
Papillary neoplasm, Breast FNA, ThinPrep®.
Marked proliferative changes are present. A fibrovascular core is difficult to discern.
40X
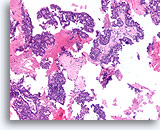
Figure 53
Papillary carcinoma, Breast FNA, Cell Block.
Low magnification of the cell block from the ThinPrep® in Figures 51-52 shows an obvious papillary configuration with a monotonous stratifying population.
10X
Figure 53
Papillary carcinoma, Breast FNA, Cell Block.
Low magnification of the cell block from the ThinPrep® in Figures 51-52 shows an obvious papillary configuration with a monotonous stratifying population.
10X
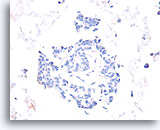
Figure 54
Papillary carcinoma, Breast FNA, Cell Block.
Absent calponin immunostaining supports the diagnosis of papillary carcinoma.
40X
Figure 54
Papillary carcinoma, Breast FNA, Cell Block.
Absent calponin immunostaining supports the diagnosis of papillary carcinoma.
40X
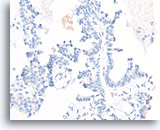
Figure 55
Papillary carcinoma, Breast FNA, Cell Block.
Absent p63 staining also helps exclude papilloma.
40X
Figure 55
Papillary carcinoma, Breast FNA, Cell Block.
Absent p63 staining also helps exclude papilloma.
40X
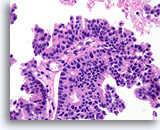
Figure 56
Papillary carcinoma, Breast FNA, Cell Block.
The papillary core is lined by freely stratifying ductal cells with an absence of myoepithelial cells.
40X
Figure 56
Papillary carcinoma, Breast FNA, Cell Block.
The papillary core is lined by freely stratifying ductal cells with an absence of myoepithelial cells.
40X
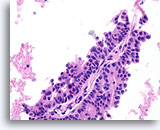
Figure 57
Papillary carcinoma, Breast FNA, Cell Block.
Note the absence of myoepithelial cells in the epithelium covering the fibrovascular core.
40X
Figure 57
Papillary carcinoma, Breast FNA, Cell Block.
Note the absence of myoepithelial cells in the epithelium covering the fibrovascular core.
40X
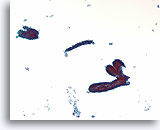
Figure 58
Fibroadenoma, Breast FNA, ThinPrep®.
Ductal cells are arranged in a « staghorn » or blunt branched duct configuration. Scattered stripped myoepithelial cells are just discernable in the background.
10X
Figure 58
Fibroadenoma, Breast FNA, ThinPrep®.
Ductal cells are arranged in a « staghorn » or blunt branched duct configuration. Scattered stripped myoepithelial cells are just discernable in the background.
10X
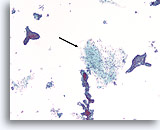
Figure 59
Fibroadenoma, Breast FNA, ThinPrep®.
Identification of stromal tissue fragments is important for a definitive diagnosis of fibroadenoma (arrow).
10X
Figure 60
Fibroadenoma, Breast FNA, ThinPrep®.
Identification of stromal tissue fragments is important for a definitive diagnosis of fibroadenoma (arrow).
10X
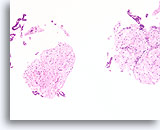
Figure 60
Fibroadenoma, Breast FNA, Cell Block.
Characteristic fibromyxoid stroma is present with benign ductal cells.
10X
Figure 60
Fibroadenoma, Breast FNA, Cell Block.
Characteristic fibromyxoid stroma is present with benign ductal cells.
10X
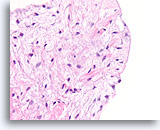
Figure 61
Fibroadenoma, Breast FNA, Cell Block.
Note the smooth surface of this stripped fibromxyoid stromal fragment and the bland morphology of the mesenchymal nuclei.
40X
Figure 61
Fibroadenoma, Breast FNA, Cell Block.
Note the smooth surface of this stripped fibromxyoid stromal fragment and the bland morphology of the mesenchymal nuclei.
40X
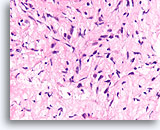
Figure 62
Phyllodes tumor, Breast FNA, Cell Block.
Compare the cellularity and variable hyperchromasia of the fibroblastic cells in this lesion to the bland uniform fibroblasts of the fibroadenoma. Since phyllodes tumors show an overgrowth of stroma, it is less common to encounter smooth surfaces that are formed by the relatively sparse epithelial component of this tumor type.
40X
Figure 62
Phyllodes tumor, Breast FNA, Cell Block.
Compare the cellularity and variable hyperchromasia of the fibroblastic cells in this lesion to the bland uniform fibroblasts of the fibroadenoma. Since phyllodes tumors show an overgrowth of stroma, it is less common to encounter smooth surfaces that are formed by the relatively sparse epithelial component of this tumor type.
40X
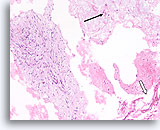
Figure 63
Phyllodes tumor, Breast FNA, Cell Block.
This section shows a thin mucoid background with individual mesenchymal cells (arrow), large pieces of ripped-out atypical stromal tissue (left) and a few broad strips of flattened ductal cells (open arrow).
10X
Figure 63
Phyllodes tumor, Breast FNA, Cell Block.
This section shows a thin mucoid background with individual mesenchymal cells (arrow), large pieces of ripped-out atypical stromal tissue (left) and a few broad strips of flattened ductal cells (open arrow).
10X
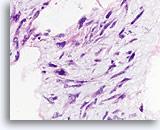
Figure 64
Phyllodes tumor, Breast FNA, Cell Block.
Nuclear atypia is prominent in this ripped-out fibromyxoid area.
60X
Figure 64
Phyllodes tumor, Breast FNA, Cell Block.
Nuclear atypia is prominent in this ripped-out fibromyxoid area.
60X
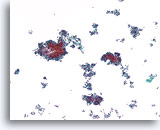
Figure 65
Ductal carcinoma, Breast FNA, ThinPrep®.
A cellular, discohesive, monotonous ductal cell population with stratification of nuclei is present.
10X
Figure 65
Ductal carcinoma, Breast FNA, ThinPrep®.
A cellular, discohesive, monotonous ductal cell population with stratification of nuclei is present.
10X
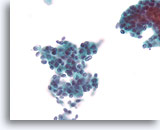
Figure 66
Ductal carcinoma, Breast FNA, ThinPrep®.
At higher magnification, randomized polarity and mild nuclear pleomorphism is evident.
40X
Figure 66
Ductal carcinoma, Breast FNA, ThinPrep®.
At higher magnification, randomized polarity and mild nuclear pleomorphism is evident.
40X
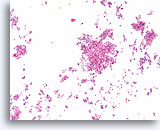
Figure 67
Ductal carcinoma, Breast FNA, Cell Block.
Low magnification of the case in Figures 65-66 shows the discohesive monotonous ductal cell population with randomized polarity and solid growth pattern.
10X
Figure 67
Ductal carcinoma, Breast FNA, Cell Block.
Low magnification of the case in Figures 65-66 shows the discohesive monotonous ductal cell population with randomized polarity and solid growth pattern.
10X
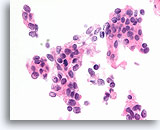
Figure 68
Ductal carcinoma, Breast FNA, Cell Block.
Randomized polarity of a cytologically atypical and freely stratifying population of ductal cells is evident.
60X
Figure 68
Ductal carcinoma, Breast FNA, Cell Block.
Randomized polarity of a cytologically atypical and freely stratifying population of ductal cells is evident.
60X
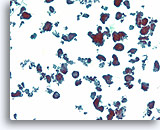
Figure 69
Ductal carcinoma, Breast FNA, ThinPrep®.
Ductal cells form a variety of detached micropapillary and hollow clusters.
10X
Figure 69
Ductal carcinoma, Breast FNA, ThinPrep®.
Ductal cells form a variety of detached micropapillary and hollow clusters.
10X
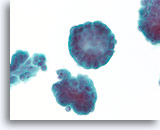
Figure 70
Ductal carcinoma, Breast FNA, ThinPrep®.
At higher magnification, the stratification of the ductal cells, random polarity, mild nuclear atypia, and absence of an apparent myoepithelial population can be seen.
60X
Figure 70
Ductal carcinoma, Breast FNA, ThinPrep®.
At higher magnification, the stratification of the ductal cells, random polarity, mild nuclear atypia, and absence of an apparent myoepithelial population can be seen.
60X
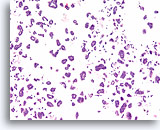
Figure 71
Ductal carcinoma, Breast FNA, Cell Block.
A corresponding tissue section shows the micropapillary (solid) and hollow glandular groups, with individual cells in the background.
10X
Figure 71
Ductal carcinoma, Breast FNA, Cell Block.
A corresponding tissue section shows the micropapillary (solid) and hollow glandular groups, with individual cells in the background.
10X
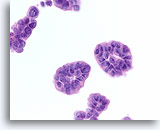
Figure 72
Ductal carcinoma, Breast FNA, Cell Block.
The tissue section from a similar field to that in Figure 70 is shown.
60X
Figure 72
Ductal carcinoma, Breast FNA, Cell Block.
The tissue section from a similar field to that in Figure 70 is shown.
60X
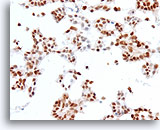
Figure 73
Ductal carcinoma, Breast FNA, Cell Block.
Estrogen receptor positivity is present in about 90% of tumor cells.
40X
Figure 73
Ductal carcinoma, Breast FNA, Cell Block.
Estrogen receptor positivity is present in about 90% of tumor cells.
40X
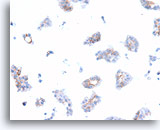
Figure 74
Ductal carcinoma, Breast FNA, Cell Block.
Dako HerceptTest immunostaining for Her2/Neu shows 1+ positivity in the case shown in Figures 69-73.
40X
Figure 74
Ductal carcinoma, Breast FNA, Cell Block.
Dako HerceptTest immunostaining for Her2/Neu shows 1+ positivity in the case shown in Figures 69-73.
40X
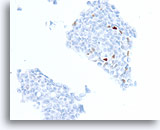
Figure 75
Ductal carcinoma, Breast FNA, Cell Block.
A different case from the previous shows about 3% of cells with positive staining for progesterone receptor.
40X
Figure 75
Ductal carcinoma, Breast FNA, Cell Block.
A different case from the previous shows about 3% of cells with positive staining for progesterone receptor.
40X
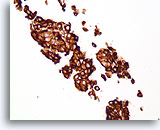
Figure 76
Ductal carcinoma, Breast FNA, Cell Block.
Dako HerceptTest immunostaining for Her2/Neu shows 3+ staining. Subsequent staining by fluorescence in situ hybridization shows amplified Her2/neu.
40X
Figure 76
Ductal carcinoma, Breast FNA, Cell Block.
Dako HerceptTest immunostaining for Her2/Neu shows 3+ staining. Subsequent staining by fluorescence in situ hybridization shows amplified Her2/neu.
40X
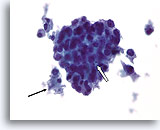
Figure 77
Ductal carcinoma, Male Breast FNA, ThinPrep®.
While gynecomastia may show extensive proliferative change, the presence of focal necrosis (arrow) and apoptotic cells (open arrow), marked atypia, and discohesion are highly worrisome.
60X
Figure 77
Ductal carcinoma, Male Breast FNA, ThinPrep®.
While gynecomastia may show extensive proliferative change, the presence of focal necrosis (arrow) and apoptotic cells (open arrow), marked atypia, and discohesion are highly worrisome.
60X
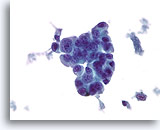
Figure 78
Ductal carcinoma, Male Breast FNA, ThinPrep®.
Marked atypia and stratification are beyond what would be expected in gynecomastia.
60x
Figure 78
Ductal carcinoma, Male Breast FNA, ThinPrep®.
Marked atypia and stratification are beyond what would be expected in gynecomastia.
60x
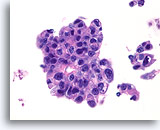
Figure 79
Ductal carcinoma, Male Breast FNA, Cell Block.
High grade nuclear atypia, necrotic debris, discohesion, and free stratification are seen.
60X
Figure 79
Ductal carcinoma, Male Breast FNA, Cell Block.
High grade nuclear atypia, necrotic debris, discohesion, and free stratification are seen.
60X
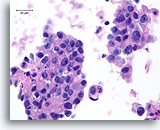
Figure 80
Ductal carcinoma, Male Breast FNA, Cell Block.
Apoptotic bodies, necrotic debris, discohesion, free stratification and high grade nuclear atypia are evident.
60X
Figure 80
Ductal carcinoma, Male Breast FNA, Cell Block.
Apoptotic bodies, necrotic debris, discohesion, free stratification and high grade nuclear atypia are evident.
60X
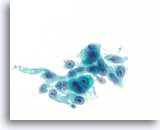
Figure 81
Ductal carcinoma, Breast FNA, ThinPrep®.
Marked nuclear atypia, stratification and disordered polarity are present.
60X
Figure 81
Ductal carcinoma, Breast FNA, ThinPrep®.
Marked nuclear atypia, stratification and disordered polarity are present.
60X
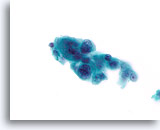
Figure 82
Ductal carcinoma, Breast FNA, ThinPrep®.
Marked pleomorphism is present.
60X
Figure 82
Ductal carcinoma, Breast FNA, ThinPrep®.
Marked pleomorphism is present.
60X
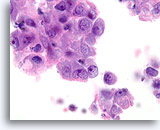
Figure 83
Ductal carcinoma, Breast FNA, Cell Block.
This section from the same case as Figures 81-82 shows a high nuclear grade, discohesive ductal population.
100X
Figure 83
Ductal carcinoma, Breast FNA, Cell Block.
This section from the same case as Figures 81-82 shows a high nuclear grade, discohesive ductal population.
100X
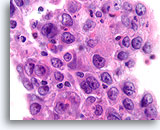
Figure 84
Ductal carcinoma, Breast FNA, Cell Block.
Admixed lymphocytes are present in this high grade ductal carcinoma.
100X
Figure 84
Ductal carcinoma, Breast FNA, Cell Block.
Admixed lymphocytes are present in this high grade ductal carcinoma.
100X
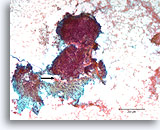
Figure 85
Breast carcinoma, Breast FNA, direct smear.
An area of desmoplastic-appearing stroma is present in the lower portion, with an irregular outline of overlying malignant cells (arrow). Although suspicious for invasion, this frequently cannot be diagnosed with certainty in cytologic preparations
10X
Figure 85
Breast carcinoma, Breast FNA, direct smear.
An area of desmoplastic-appearing stroma is present in the lower portion, with an irregular outline of overlying malignant cells (arrow). Although suspicious for invasion, this frequently cannot be diagnosed with certainty in cytologic preparations
10X
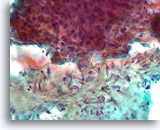
Figure 86
Breast carcinoma, Breast FNA, direct smear.
A higher magnification of the irregular interface between the ductal cells and the activated stromal tissue is shown. It is not clear whether the ductal cells are simply pushed up against the stromal tissue.
40X
Figure 86
Breast carcinoma, Breast FNA, direct smear.
A higher magnification of the irregular interface between the ductal cells and the activated stromal tissue is shown. It is not clear whether the ductal cells are simply pushed up against the stromal tissue.
40X
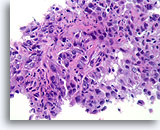
Figure 87
Invasive ductal carcinoma, Breast FNA, Cell Block.
Histologic sectioning allows invasive patterns to be identified in some ductal carcinomas [4]. The invasive pattern consists of ductal cells growing within an active stroma, without a lobular or ductal architecture. The malignant cells do not show a predictable relationship to any stromal landmarks and appear to penetrate the stroma at random angles. The features of invasion are illustrated in the following figures.
40X
Figure 87
Invasive ductal carcinoma, Breast FNA, Cell Block.
Histologic sectioning allows invasive patterns to be identified in some ductal carcinomas [4]. The invasive pattern consists of ductal cells growing within an active stroma, without a lobular or ductal architecture. The malignant cells do not show a predictable relationship to any stromal landmarks and appear to penetrate the stroma at random angles. The features of invasion are illustrated in the following figures.
40X
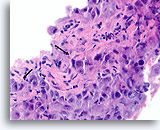
Figure 88
Ductal carcinoma, Breast FNA, Cell Block.
Note the irregular contour of the solid narrow cords of infiltrative cells (arrows).
60X
Figure 88
Ductal carcinoma, Breast FNA, Cell Block.
Note the irregular contour of the solid narrow cords of infiltrative cells (arrows).
60X
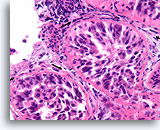
Figure 89
Ductal carcinoma in situ, Breast FNA, Cell Block.
In comparison to the irregular infiltrative groups, the four profiles of malignant ductal cells in this image are all surrounded by a smooth, dense, bright pink collagenous basement membrane. Note how the fibroblasts just outside of the basement membrane are organized with their long axis parallel to the basement membrane (arrows). Similar to breast core biopsies, the absence of invasion in this one focus does not exclude invasion in other areas.
40X
Figure 89
Ductal carcinoma in situ, Breast FNA, Cell Block.
In comparison to the irregular infiltrative groups, the four profiles of malignant ductal cells in this image are all surrounded by a smooth, dense, bright pink collagenous basement membrane. Note how the fibroblasts just outside of the basement membrane are organized with their long axis parallel to the basement membrane (arrows). Similar to breast core biopsies, the absence of invasion in this one focus does not exclude invasion in other areas.
40X
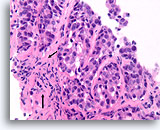
Figure 90
Ductal carcinoma, Breast FNA, Cell Block.
This focus does not show a normal lobular architecture and the stromal fibroblasts are slightly misaligned with respect to the ductal cells (arrow). However, invasion cannot be unequivocally diagnosed because the malignant ductal cells in this area form well-rounded aggregates with a smooth dense collagen layer separating them from the stroma. Similar patterns may be encountered when ductal carcinoma in situ extends into sclerosing adenosis. An absence of myoepithelial cells by IHC can help to prove that this focus is invasive [12].
40x
Figure 90
Ductal carcinoma, Breast FNA, Cell Block.
This focus does not show a normal lobular architecture and the stromal fibroblasts are slightly misaligned with respect to the ductal cells (arrow). However, invasion cannot be unequivocally diagnosed because the malignant ductal cells in this area form well-rounded aggregates with a smooth dense collagen layer separating them from the stroma. Similar patterns may be encountered when ductal carcinoma in situ extends into sclerosing adenosis. An absence of myoepithelial cells by IHC can help to prove that this focus is invasive [12].
40x
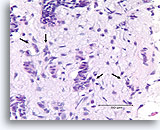
Figure 91
Invasive ductal carcinoma, Breast FNA, Cell Block.
One residual, probably non-invasive, duct is seen in the upper-most part of the Figure (open arrow). Invasion can be diagnosed in the other irregular, solid, thin clusters of malignant ductal cells that dissect the activated stromal tissue randomly (arrows).
40x
Figure 91
Invasive ductal carcinoma, Breast FNA, Cell Block.
One residual, probably non-invasive, duct is seen in the upper-most part of the Figure (open arrow). Invasion can be diagnosed in the other irregular, solid, thin clusters of malignant ductal cells that dissect the activated stromal tissue randomly (arrows).
40x
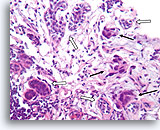
Figure 92
Invasive ductal carcinoma, Breast FNA, Cell Block.
In this area, breast cancer cells (arrows) are infiltrating amongst some native ducts (open arrows).
40X
Figure 92
Invasive ductal carcinoma, Breast FNA, Cell Block.
In this area, breast cancer cells (arrows) are infiltrating amongst some native ducts (open arrows).
40X
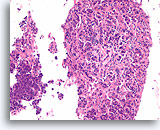
Figure 93
Invasive ductal carcinoma, Breast FNA, Cell Block.
Nearly the whole diameter of a 22 gauge FNA needle was captured in this section. An obvious infiltrative pattern is evident at low magnification.
20X
Figure 93
Invasive ductal carcinoma, Breast FNA, Cell Block.
Nearly the whole diameter of a 22 gauge FNA needle was captured in this section. An obvious infiltrative pattern is evident at low magnification.
20X
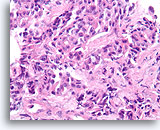
Figure 94
Invasive ductal carcinoma, Breast FNA, Cell Block.
Irregular strands and cords of ductal cells penetrate the stromal tissue haphazardly.
40X
Figure 94
Invasive ductal carcinoma, Breast FNA, Cell Block.
Irregular strands and cords of ductal cells penetrate the stromal tissue haphazardly.
40X
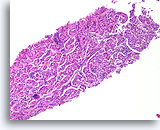
Figure 95
Invasive ductal carcinoma, Breast FNA, Cell Block.
Nests of ductal cells are fairly well-rounded, but the pattern does not suggest the organization of normal native lobules, even when distorted by sclerosing adenosis.
10X
Figure 95
Invasive ductal carcinoma, Breast FNA, Cell Block.
Nests of ductal cells are fairly well-rounded, but the pattern does not suggest the organization of normal native lobules, even when distorted by sclerosing adenosis.
10X
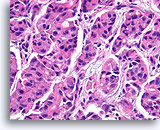
Figure 96
Invasive ductal carcinoma, Breast FNA, Cell Block.
At higher magnification, no myoepithelial population is observed, helping to exclude colonization of sclerosing adenosis.
40x
Figure 96
Invasive ductal carcinoma, Breast FNA, Cell Block.
At higher magnification, no myoepithelial population is observed, helping to exclude colonization of sclerosing adenosis.
40x
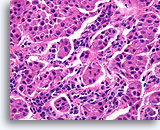
Figure 97
Invasive ductal carcinoma, Breast FNA, Cell Block.
In other areas of the case shown in Figures 95-96, the shapes of the nests varies from broad to small angular aggregates. The pattern is too random for colonization of sclerosing adenosis, and no basement membrane or myoepithelial cells are seen.
40x
Figure 97
Invasive ductal carcinoma, Breast FNA, Cell Block.
In other areas of the case shown in Figures 95-96, the shapes of the nests varies from broad to small angular aggregates. The pattern is too random for colonization of sclerosing adenosis, and no basement membrane or myoepithelial cells are seen.
40x
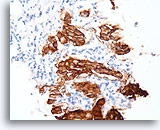
Figure 98
Invasive ductal carcinoma, Breast FNA, Cell Block.
Dako HercepTest 3+ staining for Her2/Neu is observed within the invasive tumor. For some applications, it may be necessary to distinguish Her2/neu positivity of the invasive tumor from that of any in situ component.
40x
Figure 98
Invasive ductal carcinoma, Breast FNA, Cell Block.
Dako HercepTest 3+ staining for Her2/Neu is observed within the invasive tumor. For some applications, it may be necessary to distinguish Her2/neu positivity of the invasive tumor from that of any in situ component.
40x
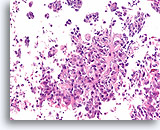
Figure 99
Invasive ductal carcinoma, Breast FNA, Cell Block.
Invasion is obvious in this high grade ductal carcinoma.
20X
Figure 99
Invasive ductal carcinoma, Breast FNA, Cell Block.
Invasion is obvious in this high grade ductal carcinoma.
20X
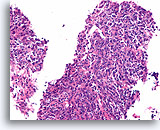
Figure 100
Invasive poorly differentiated carcinoma, Breast FNA, Cell Block.
This infiltrating high grade carcinoma has a heavy admixture of lymphocytes.
20X
Figure 100
Invasive poorly differentiated carcinoma, Breast FNA, Cell Block.
This infiltrating high grade carcinoma has a heavy admixture of lymphocytes.
20X
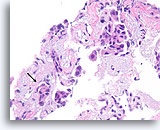
Figure 101
Ductal carcinoma, Breast FNA, Cell Block.
Though fragmented, at least one irregular nest of ductal cells without an apparent myoepithelial layer can be seen within desmoplastic stromal tissue (arrow), highly suspicious for invasion.
40x
Figure 101
Ductal carcinoma, Breast FNA, Cell Block.
Though fragmented, at least one irregular nest of ductal cells without an apparent myoepithelial layer can be seen within desmoplastic stromal tissue (arrow), highly suspicious for invasion.
40x
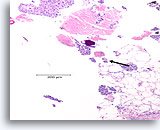
Figure 102
Ductal carcinoma, Breast FNA, Cell Block.
Necrotic debris and a microcalcification are seen (arrow), with detached solid masses of high-grade ductal carcinoma cells. This pattern is typical of comedo carcinoma in situ. An area of desmoplastic stroma is just evident at the lower right (shown in Figure 103).
10x
Figure 102
Ductal carcinoma, Breast FNA, Cell Block.
Necrotic debris and a microcalcification are seen (arrow), with detached solid masses of high-grade ductal carcinoma cells. This pattern is typical of comedo carcinoma in situ. An area of desmoplastic stroma is just evident at the lower right (shown in Figure 103).
10x
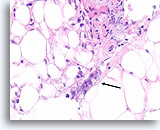
Figure 103
Ductal carcinoma, Breast FNA, Cell Block.
At the edge of the desmoplastic stroma of Figure 102 is an irregular solid tongue of tumor cells, suspicious for invasion (arrow).
40X
Figure 103
Ductal carcinoma, Breast FNA, Cell Block.
At the edge of the desmoplastic stroma of Figure 102 is an irregular solid tongue of tumor cells, suspicious for invasion (arrow).
40X

Figure 104
Lobular carcinoma, Breast FNA, Cell Block.
Lobular carcinoma cells seem embedded in the stromal tissue giving the impression of hypercellularity. This may be a reason why cytology preparations of lobular carcinoma often show few malignant cells.
20X
Figure 104
Lobular carcinoma, Breast FNA, Cell Block.
Lobular carcinoma cells seem embedded in the stromal tissue giving the impression of hypercellularity. This may be a reason why cytology preparations of lobular carcinoma often show few malignant cells.
20X
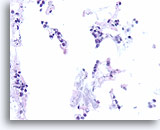
Figure 105
Lobular carcinoma, Breast FNA, Cell Block.
« Indian filing » of minimally atypical sparse cells stuck on and within the stromal fragments is seen. Note the characteristic targetoid intracytoplasmic vacuoles of lobular neoplasia.
40X
Figure 105
Lobular carcinoma, Breast FNA, Cell Block.
« Indian filing » of minimally atypical sparse cells stuck on and within the stromal fragments is seen. Note the characteristic targetoid intracytoplasmic vacuoles of lobular neoplasia.
40X
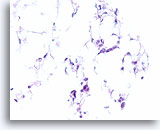
Figure 106
Invasive lobular carcinoma, Breast FNA, Cell Block.
Thin cords of lobular carcinoma cells infiltrate the adipose tissue.
40X
Figure 106
Invasive lobular carcinoma, Breast FNA, Cell Block.
Thin cords of lobular carcinoma cells infiltrate the adipose tissue.
40X
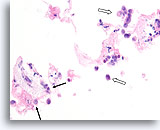
Figure 107
Invasive lobular carcinoma, Breast FNA, Cell Block.
Sparse detached lobular carcinoma cells are present (open arrow). Focally, they infiltrate the stromal tissue (arrows).
40x
Figure 107
Invasive lobular carcinoma, Breast FNA, Cell Block.
Sparse detached lobular carcinoma cells are present (open arrow). Focally, they infiltrate the stromal tissue (arrows).
40x
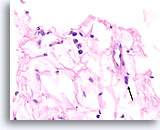
Figure 108
Lobular carcinoma, Breast FNA, Cell Block.
This field shows a few detached lobular carcinoma cells intermingled with the adipose tissue. Two other cells are suspicious for invasion within the tissue (arrow).
40X
Figure 108
Lobular carcinoma, Breast FNA, Cell Block.
This field shows a few detached lobular carcinoma cells intermingled with the adipose tissue. Two other cells are suspicious for invasion within the tissue (arrow).
40X
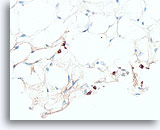
Figure 109
Lobular carcinoma, Breast FNA, Cell Block.
A pan-keratin immunostain can be helpful to disclose the sparse infiltrating lobular carcinoma cells.
40X
Figure 109
Lobular carcinoma, Breast FNA, Cell Block.
A pan-keratin immunostain can be helpful to disclose the sparse infiltrating lobular carcinoma cells.
40X
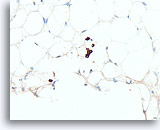
Figure 110
Lobular carcinoma, Breast FNA, Cell Block.
A cluster of 4 lobular carcinoma cells and a few other individual lobular carcinoma cells are highlighted with a pankeratin immunostain.
40X
Figure 110
Lobular carcinoma, Breast FNA, Cell Block.
A cluster of 4 lobular carcinoma cells and a few other individual lobular carcinoma cells are highlighted with a pankeratin immunostain.
40X
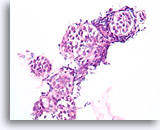
Figure 111
Lobular neoplasia, Breast FNA, Cell Block.
An in-situ growth pattern of lobular neoplasia is difficult or impossible to identify in cytology preparations. This cluster of about 5 acini is partially distended by the characteristic loose « bag of marbles » pattern of lobular neoplasia, without formation of a glandular lumen.
40X
Figure 111
Lobular neoplasia, Breast FNA, Cell Block.
An in-situ growth pattern of lobular neoplasia is difficult or impossible to identify in cytology preparations. This cluster of about 5 acini is partially distended by the characteristic loose « bag of marbles » pattern of lobular neoplasia, without formation of a glandular lumen.
40X
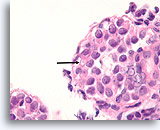
Figure 112
Lobular neoplasia, Breast FNA, Cell Block.
Higher magnification shows a characteristic targetoid cytoplasmic vacuole (arrow)
100X
Figure 112
Lobular neoplasia, Breast FNA, Cell Block.
Higher magnification shows a characteristic targetoid cytoplasmic vacuole (arrow)
100X
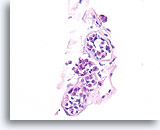
Figure 113
Lobular neoplasia, Breast FNA, Cell Block.
This case shows three acini partly distended by lobular neoplasia.
40X
Figure 113
Lobular neoplasia, Breast FNA, Cell Block.
This case shows three acini partly distended by lobular neoplasia.
40X