Specimen Adequacy
James Linder, MD
In order for a Pap sample to be properly interpreted, it must contain adequate numbers of squamous epithelial cells. The terminology from the Bethesda 2014 conference recommends that Pap tests be categorized either as “satisfactory for evaluation” or “unsatisfactory for evaluation”. The “satisfactory but limited by” category that was a component of previous Bethesda schemes was eliminated. A description of the presence or absence of endocervical/transformation zone component and any other quality factors may be included as a descriptive component of “Satisfactory”, but not “limit” the adequacy. At least 10 well-preserved endocervical or squamous metaplastic cells should be observed to report that a transformation zone component is present. If the specimen shows a high-grade lesion or cancer, it is not necessary to report the presence/absence of transformation component.
SATISFACTORY FOR EVALUATION
Generally, a minimum of 5,000 well-visualized/preserved squamous cells are needed for a specimen to be considered satisfactory, with the following caveats:
- It is recognized that strict objective criteria are not applicable in every case. For example, slides with cell clustering, atrophy, or cytolysis are technically difficult to count, and laboratories should apply professional judgment and employ hierarchical review when evaluating these slides.
- Not every specimen requires a count to be employed to assess cellularity. If the cytotechnologist feels there may be less than 5000 well-visualized/ preserved squamous cells present a counting scheme as follows may be utilized.
Any specimen with abnormal cells is by definition satisfactory for evaluation.
If it is necessary to count squamous cells in a ThinPrep slide: 1. A minimum of 10 fields should be counted along a diameter that includes the center of the preparation. The average cell number per microscopic field to achieve 5000 cells is shown in the following table:
|
PREP DIAM mm
AREA |
20
314.2 |
| FN20 eyepiece/10X objective |
# Fields@FN20 10X
# Cells/field for 5K Total |
100
50.0 |
| FN20 eyepiece/40x objective |
# Fields@FN20 40X
# Cells/field for 5K Total |
1600
3.1 |
| FN22 eyepiece/10x objective |
# Fields@FN22 10X
# Cells/field for 5K Total |
82.6
60.5 |
| FN22 eyepiece/40x objective |
# Fields@FN22 40X
# Cells/field for 5K Total |
1322
3.8 |
For individuals using oculars not shown, the formula is:
Number of Cells Required per Field =5000 / (Area of Circle / Area of Ocular)
The diameter of an ocular or microscopic field in millimeters is the field number of the eyepiece divided by the magnification of the objective. The area of the field can then be determined by the formula for the area of a circle [pi X (Radius squared)].
For slides processed with the ThinPrep® Imaging System, adequacy should be determined as outlined in the Operator’s Manual.
According to Bethesda 2014, additional information on the meaning of adequacy qualifiers and any implications for patient follow-up may be provided optionally in an educational note. Unsatisfactory Paps that are processed and evaluated require considerable time and effort. While such specimens cannot exclude an epithelial lesion, some helpful information (presence of infectious organisms, etc) may be provided that can help direct further patient management. Suggested wording from Bethesda 20141 to clarify reports is as follows:
A) Rejected Pap:
Specimen rejected (not processed) because ____ (specimen not labeled, slide broken, etc.)
B) Fully evaluated unsatisfactory Pap:
Specimen processed and examined, but unsatisfactory for evaluation of epithelial abnormality because of ____ (insufficient squamous epithelium due to blood, etc.) Additional comments/recommendations, as appropriate
OBSCURING FACTORS
Specimens with >75% of cells obscured should be termed unsatisfactory (assuming no abnormal cells are present). When 50-75% of cells are obscured, a statement describing the specimen as partially obscured should follow the satisfactory term.
The photomicrographs contained in this atlas will display a wide variety of cellular specimens containing adequate squamous and glandular material. For the purpose of this topic, photomicrographs containing the minimum number of cells for adequacy are included as well as examples of transformation zone components.

Reminder: You may click on any slide image for an enlarged view.

Satisfactory
Pictured is a representative 10X field containing 60 well visualized/ preserved squamous cells from an adequate ThinPrep slide.
Satisfactory
Pictured is a representative 10X field containing 60 well visualized/ preserved squamous cells from an adequate ThinPrep slide.
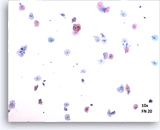
Satisfactory
Pictured is a representative 10X field containing 50 well visualized/ preserved squamous cells from an adequate ThinPrep slide.
Satisfactory
Pictured is a representative 10X field containing 50 well visualized/ preserved squamous cells from an adequate ThinPrep slide.

Unsatisfactory
Pictured is a representative 10X field containing 40 well visualized/ preserved squamous cells from an unsatisfactory ThinPrep slide.
Unsatisfactory
Pictured is a representative 10X field containing 40 well visualized/ preserved squamous cells from an unsatisfactory ThinPrep slide.
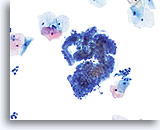
Transformation zone component
Endocervical cells demonstrating both picket fence and honeycomb arrangements compose a transformation zone component. 20X
Transformation zone component
Endocervical cells demonstrating both picket fence and honeycomb arrangements compose a transformation zone component. 20X
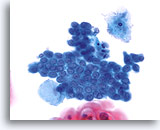
Transformation zone component
Smaller, cuboidal endocervical cells with nuclei located on top of their cytoplasm provide a transformation zone component. 40X
Transformation zone component
Smaller, cuboidal endocervical cells with nuclei located on top of their cytoplasm provide a transformation zone component. 40X
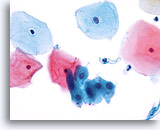
Transformation zone component
Squamous metaplastic cells represent a transformation zone component. 40X
Transformation zone component
Squamous metaplastic cells represent a transformation zone component. 40X
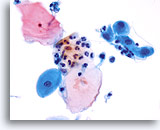
Transformation zone component
Squamous metaplasia represents the transformation zone. 40X
Transformation zone component
Squamous metaplasia represents the transformation zone. 40X
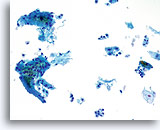
Limiting factor
Inflammatory cells limiting the squamous cell component of the specimen. 20X
Limiting factor
Inflammatory cells limiting the squamous cell component of the specimen. 20X
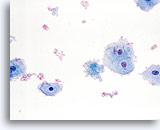
Limiting factor
Blood limiting the adequacy of the squamous cell component. 40X
Limiting factor
Blood limiting the adequacy of the squamous cell component. 40X
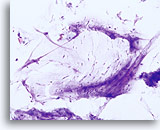
Limiting factor
Lubricant limiting the adequacy of the squamous cell component. 20X
Limiting factor
Lubricant limiting the adequacy of the squamous cell component. 20X
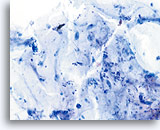
Limiting factor
Mucus limiting the adequacy of the squamous cell component. 20X
Limiting factor
Mucus limiting the adequacy of the squamous cell component. 20X
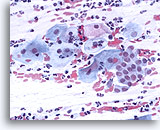
Conventional Pap smear
Conventional Pap smear with transformation zone and squamous components. 40X
Conventional Pap smear
Conventional Pap smear with transformation zone and squamous components. 40X
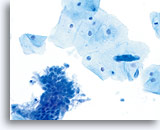
ThinPrep Pap Test
Same patient sample prepared with the ThinPrep method. Squamous and glandular components are present. 40X
ThinPrep Pap Test
Same patient sample prepared with the ThinPrep method. Squamous and glandular components are present. 40X
In summary, regardless of how the specimen is prepared, following the guidelines for the proper collection of a Papanicolaou test as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) is essential to an adequate sampling and accurate diagnosis of the patient’s condition. If inflammatory debris, blood or mucus is accumulated on the cervix and not properly
removed, the accumulation will be collected and appear on the slide. If lubricant is used it will interfere with the transfer of cells to the glass slide. Consequently the slide will not represent the condition of the patient and may lead to a misdiagnosis.
Proper collection, along with complete rinsing of the collection device(s) in a timely manner, will allow for a representative sample of the patient’s epithelium to be transferred to the glass slide during processing and an improvement in the detection of disease of the cervix.
SUGGESTED READINGS:
- Bernstein SJ, et al: Liquid-based cervical cytologic smear study and conventional Papanicolaou smears: A metaanalysis of prospective studies comparing cytologic diagnosis and sample adequacy. Am J Obstet Gynecol 2001;185:308-17.
- Bethesda 2014 Post workshop recommendations.
www.bethesda2014.cancer.gov.
- Corkill M, et al: Specimen adequacy of ThinPrep sample preparations in a direct-to-vial study. Acta Cytol 1997;41:39-44.
- Darragh TM, et al: Comparison of conventional cytologic smears and ThinPrep preparations from the anal canal. Acta Cytol 1997; 41: 1167-70.
- Diaz-Rosario LA, Kabawat SE: Performance of a fluid-based, thin-layer Papanicolaou smear method in the clinical setting of an independent laboratory and an outpatient screening population in New England. Arch Pathol Lab Med 1999;123(9):817-21.
- Guidos BJ, Selvaggi SM: Use of the ThinPrep Pap Test in clinical practice. Diagn Cytopathol 1999;20:70-73.
- Haroon S, et al: Reproducibility of cervicovaginal ThinPrep® cellularity assessment. Diagn Cytolpathol 2002 jan;26(1):19-21.
- Henry JA, Wadehra V: Influence of smear quality on the rate of detecting significant cervical cytologic abnormalities. Acta Cytol 1996; 40: 529-35.
- Kivlahan C, Ingram E: Papanicolaou smears without endocervical cells. Are they inadequate? Acta Cytol 1986; 30: 258-60.
- Mintzer MP, et al: The effect of the quality of Papanicolaou smears on the detection of cytologic abnormalities. Cancer Cytopathol 1999; 87: 113-7.
- Mitchell HS: Longitudinal analysis of histologic high-grade disease after negative cervical cytology according to endocervical status. Cancer 2001;Aug 25;93(4):237-40.
- Papillo J, et al: Evaluation of the ThinPrep Pap Test in clinical practice: a seven-month 16,314-case experience in northern Vermont. Acta Cytol 1998;42:204-208.
- Renshaw AA, et al: Accuracy and reproducibility of estimating the adequacy of the squamous component of cervicovaginal smears. Am J Clin Path 1999; 111: 38-42.
- Selvaggi SM, Guidos BJ: Specimen adequacy and the ThinPrep Pap Test: The endocervical component. Diagn Cytopathol 2000; 23(1):23-26.
- Sherman ME, et al: Cytologic diagnosis of anal intraepithelial neoplasia using smears and Hologic Thin-Preps. Mod Path 1995; 8: 270-74.
- Spires SE, et al: Assessment of cervicovaginal smear adequacy. The Bethesda system guidelines and reproducibility. Am J Clin Path 1994; 102: 354-9.
- Timmerman TG, et al: Objective criteria to determine cellularity in the ThinPrep Papanicolaou test. Acta Cytol 1998; 42: 1242.
- Valente P et al: The determination of Papanicolaou smear adequacy using a semiquantitative method to evaluate cellularity. Diagn Cytopathol 1991; 7: 576-80.













