GYN Atlas - Epithelial Cell Abnormalities - Squamous Cells
ACKNOWLEDGEMENTS
Suzanne M. Werneke, BA, CT(ASCP)
I would like to thank Kurt Douglass, CT(ASCP) for his initial efforts to bring this project to completion and for the original version of the ThinPrep Morphology Reference Manual, which is still in use today. Thanks also go to Mike LeDonne, CT(ASCP) for many of his early digital images used in this atlas.
I would personally like to thank all of our guest editors who worked diligently to meet our time lines. We have learned much from each of these pathologists and cytotechnologists and greatly appreciate their enthusiasm and admire their passion for the field of cytology.
Finally, many thanks go to Charlotte L. Brahm, CT(ASCP), Managing Editor, whose name appears nowhere else in this atlas, but who worked tirelessly to bring it to fruition.
Contributors
Raheela Ashfaq, MD
Professor, Department of Pathology, University of Texas Southwestern Medical Center, and Director of Cytopathology and Oncodiagnostic Laboratory, Parkland Health and Hospital, Dallas, TX
R. Marshall Austin, MD, PhD
Medical Director and Director of Cytopathology and Gynecologic Pathology Services, Coastal Pathology Associates, Charleston, SC
John M. Bauer, MD
Medical Director, Piedmont Pathology, Incorporated, Hickory, NC
Diane D. Davey, MD
Professor of Pathology and Laboratory Medicine, Director of Cytopathology and Bone Marrow Service, Director of Cytopathology Fellowship Program, University of Kentucky, Lexington, KY
Luis A. Diaz-Rosario, MD
Medical Director, Anatomic Pathology, Quest Diagnostics, Incorporated, Tampa, FL
Martha L. Hutchinson, MD, PhD
Associate Professor Pathology, Brown University, Providence, RI
Deanna K. Iverson, MHS, SCT(ASCP), HTL
Pathology Manager, Palo Alto Medical Foundation, Palo Alto, CA
Joyce E. Johnson, MD
Associate Professor, Department of Pathology, Director Division of Education, Vanderbilt University Medical Center, Nashville, TN
James Linder, MD
Medical Director, Hologic, Inc., Marlborough, MA; Professor, Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE
Jacalyn L. Papillo, BS, CT(ASCP)
Cytopathology Manager, Fletcher Allen Healthcare, Burlington, VT
Pamela Smith Piraino, CT(ASCP)
Clear Path Diagnostics, Syracuse, NY
Richard A. Smith, MD, PhD
Chief of Pathology, Sturdy Memorial Hospital, Attleboro, MA
Zahniser, David, PhD
President, Diagnostic Vision Incorporated, Wellesley, MA
INTRODUCTION
This atlas is designed to assist cytotechnologists and pathologists develop and refine their proficiency in the interpretation of gynecologic cytology using the ThinPrep® Pap Test. The early research and development of the ThinPrep Pap at Hologic, Inc. established a foundation for the comparisons made to the conventional Pap smear. Since that time, other cytotechnologists and pathologists in clinical practice throughout the world have helped to refine that foundation. This reference atlas is meant to serve as an educational tool and ongoing reference for users of the ThinPrep Pap Test.
This atlas is designed to assist cytotechnologists and pathologists develop and refine their proficiency in the interpretation of gynecologic cytology using the ThinPrep® Pap Test. The early research and development of the ThinPrep Pap at Hologic, Inc. established a foundation for the comparisons made to the conventional Pap smear. Since that time, other cytotechnologists and pathologists in clinical practice throughout the world have helped to refine that foundation. This reference atlas is meant to serve as an educational tool and ongoing reference for users of the ThinPrep Pap Test.
The organization of the atlas corresponds to The Bethesda System as it was developed at the National Cancer Institute, and summarized in a publication by Robert J. Kurman and Diane Solomon, entitled The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses: Definitions, Criteria and Explanatory Notes for Terminology and Specimen Adequacy (New York: Springer-Verlag, 1994). Updates to include Bethesda 2001 nomenclature have been included. The book entitled The Art and Science of Cytopathology by Dr. Richard DeMay has also been referenced for cytologic criteria pertaining to atypia, dysplasia and more severe lesions.
For each diagnostic category, images illustrate ThinPrep Pap Test (TPPT) morphology, the corresponding appearance of the conventional Pap smear, and the key look-alike entities. All photographs in this atlas represent ThinPrep slides, unless otherwise noted. The photographs are intended to illustrate typical findings on the ThinPrep sample preparations and may be subject to individual interpretation.
OVERVIEW OF THE
THINPREP PREPARATION METHOD
The ThinPrep® System for Pap specimens is FDA approved as a replacement to the conventional method for detecting atypical cells, cervical cancer and its precursor lesions as well as other cytologic categories as defined by The Bethesda System. The ThinPrep Pap Test has been proven to be significantly more effective than the conventional Pap smear for the detection of Low-grade Squamous Intraepithelial Lesions (LSIL) and more severe lesions in a variety of patient populations, including a 59.7% increase in the detection of HSIL and higher grade lesions in a direct-to-vial HSIL study. Specimen quality is also significantly improved over that of the conventional Pap smear preparation.
The ThinPrep® System for Pap specimens is FDA approved as a replacement to the conventional method for detecting atypical cells, cervical cancer and its precursor lesions as well as other cytologic categories as defined by The Bethesda System. The ThinPrep Pap Test has been proven to be significantly more effective than the conventional Pap smear for the detection of Lo
w-grade Squamous Intraepithelial Lesions (LSIL) and more severe lesions in a variety of patient populations, including a 59.7% increase in the detection of HSIL and higher grade lesions in a direct-to-vial HSIL study. Specimen quality is also significantly improved over that of the conventional Pap smear preparation.
Optimal sample collection and preparation are the most important factors in improving the accuracy of the Pap test. Differences in the procedures used to prepare conventional smears and ThinPrep Pap Test specimens translates into differences in the microscopic appearance between conventional smears and ThinPrep samples. Correct interpretation of ThinPrep Pap Test slides requires an understanding of the ThinPrep preparation method. This is especially important with respect to qualities of the sample that effect the preservation and presentation of the specimen. For this reason, the ThinPrep preparation method is summarized below. The automated process in the ThinPrep processor includes dispersion of the sample, precision filtration to collect cells onto a membrane, and transfer to a glass microscope slide.
ThinPrep Processor:Principles of Operation
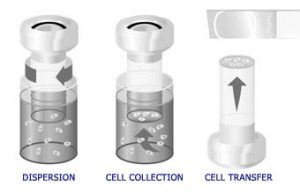
The ThinPrep process begins with the patient’s gynecologic sample being collected by the clinician using either a broom-like device or a brush/plastic spatula combination. The device is then rinsed in the specimen vial containing PreservCyt® Solution. The ThinPrep sample vial is then capped, labeled, and sent to a laboratory equipped with a ThinPrep Processor.
At the laboratory, the sample vial is matched to the corresponding cytology requisition form and a cytology number is assigned to the form, the vial, and a ThinPrep microscope slide. The vial and matching slide are placed into the ThinPrep Processor along with a disposable gynecologic filter cylinder (clear filter), and the operator selects the appropriate slide sequence. The instrument homogenizes the sample by spinning either the filter (T2000) or the vial (T3000) creating shear forces in the fluid that are strong enough to separate randomly joined material, break up blood, mucus and non-diagnostic debris while keeping true cell clusters intact. If rinsed properly, all the cells collected go into solution instead of being left on the device and discarded after making a conventional Pap smear. This fact, along with the randomization of the cell sample during the spinning phase, allows for a representative cellular sample to be transferred to the slide and a more accurate reflection of the status of the patient’s epithelium.
The cells are then collected onto the membrane of the TransCyt Filter by applying a gentle vacuum across the filter membrane to aspirate fluid through the membrane. As the material in the specimen vial collects on the filter membrane, the pores of the membrane become blocked thereby triggering the processor to stop aspirating. The flow rate through the TransCyt Filter is constantly monitored to minimize the variability of the specimens.
When the processor stops the filtration step, the filter cylinder is withdrawn from the sample vial and partially rotated to evacuate the filtrate into the waste bottle. The filter cylinder is then rotated up to the glass slide. Slight air pressure is applied to the filter to affect the transfer of the cells from the filter to the slide. Due to the natural adhesion properties of cells and the natural electrochemical properties of the ThinPrep slides, the cells display a higher affinity for the glass slide than for the filter membrane and a thin-layer of cells is transferred to the glass slide in a 20mm diameter circle. Once cell transfer is complete, the slide is removed from contact with the filter cylinder and ejected automatically into a fixative bath. It is then removed by the operator and placed into a staining rack (T2000) for subsequent routine Papanicolaou staining and coverslipping. With the T3000 processor, the slide is fixed by the instrument with a mist of fixative solution and placed into a staining rack for subsequent routine Papanicolaou staining and coverslipping.
References
- Hutchinson ML, et al: Homogeneous sampling accounts for the increased diagnostic accuracy using the ThinPrep® Processor. Am J of Clin Pathol 1994; 101:215-219.
- ThinPrep® 2000 System Package Insert, Hologic, Inc., 2001.
- TP® 2000 Operator’s Manual, Rev G, Hologic, Inc., 2000.
- TP® 3000 Operator’s Manual, Rev C, Hologic, Inc., 2000-2001.
Scientific Foundations
David Zahniser, PhD
The false-negative Pap smear is a major quality issue facing the cytology laboratory. The failure to detect cervical disease in women is linked to either sampling errors or screening errors. Sampling errors account for the majority of false negative Pap smears, ranging from 60-80%. Screening and interpretive errors account for the remaining errors. Historically, sampling errors were ascribed to the clinician not properly sampling the lesion at the time of cell harvest. It is thought that the lesion is either too small, or may have been high in the endocervical canal. It has become apparent that sampling errors play an important role in limiting the sensitivity of the Pap smear. Early work in the development of the ThinPrep® 2000 System helped to uncover inherent pitfalls in the preparation of the conventional Pap smear and further defined sampling errors. Studies now show that sampling errors are not necessarily physician dependent, but more specifically associated with limitations in the manual smearing technique.
The false-negative Pap smear is a major quality issue facing the cytology laboratory. The failure to detect cervical disease in women is linked to either sampling errors or screening errors. Sampling errors account for the majority of false negative Pap smears, ranging from 60-80%. Screening and interpretive errors account for the remaining errors. Historically, sampling errors were ascribed to the clinician not properly sampling the lesion at the time of cell harvest. It is thought that the lesion is either too small, or may have been high in the endocervical canal. It has become apparent that sampling errors play an important role in limiting the sensitivity of the Pap smear. Early work in the development of the ThinPrep® 2000 System helped to uncover inherent pitfalls in the preparation of the conventional Pap smear and further defined sampling errors. Studies now show that sampling errors are not necessarily physician dependent, but more specifically associated with limitations in the manual smearing technique.
The origins of ThinPrep technology date back to when various research efforts to develop a quantitative system to analyze the Pap smear were underway. During the 1960’s, digital computers made it possible to build scanning systems. From the start, computer imaging systems were limited by the inconsistent manual preparations. It was evident that traditional cervical smears were too complicated for analysis by an automated system. Much research went into developing preparation devices for machine-readable preparations. First generation automated preparation systems were being designed to achieve a single cell population with no clusters to be suitable for analysis by the primitive computers. To disaggregate these sheets and clusters of cells, various mechanical means were tested. Cervical scrapings collected into solution were subjected to vigorous mechanical action. The most successful preparation devices used syringing action, pumping the cell sample through a large gauge syringe needle, to achieve a single cell population. These devices were successful at presenting the computer with simplified cell preparations. Unfortunately, successful mono-dispersion was accompanied by cell loss and tissue architecture being destroyed in the process, compromising the human interpretation. Work in the Netherlands addressed some of the problems related to syringing. They approached the dispersion step with the use of a cylinder rotating at high speeds within the cell suspension. The thought was that as the cylinder is rotated in the vial, shear forces, similar to the syringing action, are gained. By controlling the speed of the dispersion cylinder the degree of dispersion could be manipulated. The current ThinPrep Processors incorporate the cylinder dispersion technique using a much lower dispersion speed to preserve the integrity of the natural cell bonds. In addition, modifications to the collection solution have been made to further optimize the sample preparation.
A filtration method was also under development in conjunction with the dispersion step. The quality of the sample could be improved further by limiting non-diagnostic background elements. Care was taken that no essential information originally present in the sample be lost. A plastic filter membrane was selected to concentrate the cellular material from the fluid suspension and limit such artifacts by simple filtration. The process used to produce these filters results in a random distribution of pores with a uniform size.
The preparation device required an additional step to deposit the cells in suspension onto a glass microscope slide. Numerous techniques were studied to optimize the deposit of cells onto the slide so as to be suitable for both human and computer analysis. The use of the filter membrane allowed the transfer of cells to the slide by pressing the filter and cells against the slide resulting in a thin, uniform layer of cells with even distribution and familiar cell morphology.
Renewed interest in Pap smear automation has been fueled by a shortage in the cytotechnologist workforce and increased Pap smear litigation. Numerous commercially available computer-screening instruments are on the market. However, even as computer technology has advanced, the need for consistent slide preparation continues. ThinPrep technology gained momentum to address the slide preparation limitations and non-clinical and clinical studies to support ThinPrep technology have helped to better understand the Pap smear limitations.
References
- Linder J and Zahniser: ThinPrep Papanicolaou testing to reduce false-negative cervical
- Arch Pathol Lab Med; 1998; 122:139-144.
