THYROID
Helen H. Wang, MD, DrPH
Introduction
Thyroid nodules are common clinical findings with an estimated annual incidence rate of 4-8%.[1] At any given time, between 10 and 20 million Americans have clinically detectable thyroid nodules.[1] Thyroid fine needle aspiration (FNA) is the initial test in the management of most patients with a thyroid nodule because it is safe and inexpensive and provides better selection of patients for surgery than any other test.[2] Therefore, thyroid FNA is easily the most common type of FNA specimens in a cytology laboratory. Although it has been widely accepted and practiced since the 1970s, the processing and reporting of thyroid FNA’s are still controversial.
Specimen Collection and Processing
Thyroid FNA is obtained under the guidance of palpation or ultrasound, depending on the size of the lesion. Both are processed the same way, i.e. to either make conventional direct smears from the aspirate or to rinse the aspirate into a preservative to make a liquid-based preparation. Two studies that have compared the accuracy of ThinPrep® preparations versus conventional smears for thyroid FNA agreed that these two methods had comparable accuracy for thyroid neoplasms,[3, 4] although one of them showed ThinPrep preparations to have lower overall correlation with histologic diagnosis than direct smears, especially in detecting chronic lymphocytic thyroiditis (62% by ThinPrep versus 92% by direct smears).[4] In addition, some authors have suggested that ThinPrep preparations do not detect diffuse or watery colloid.[3, 5] In study by Tulecke, et al. “tissue-paper-like material” on ThinPrep was found to be associated with abundant colloid on histology and probably represents watery colloid. [6] Overall, colloid seems to be present in less quantity on ThinPrep than on conventional smears. However, ThinPrep is superior to conventional smears, whether fixed in alcohol or left to air-dry, in demonstrating nuclear features that are crucial in the diagnosis of papillary carcinoma. ThinPrep on the other hand appears to break up follicles and presents follicular cells in sheets, groups or even singly more readily than on conventional smears.
Reporting
Many reporting schemes have been proposed and used in the literature. We have found a reporting scheme based on the probability of finding carcinoma on histology to be helpful to both pathologists and clinicians.
Positive for malignancy
Specimens in this category represent those cases in which a malignancy is found on resections virtually 100% of the time. Papillary carcinoma is the most common malignancy of the thyroid and has specific nuclear features that are easily identified on cytologic samples. Other malignancies that can be diagnosed on cytology with high accuracy include medullary carcinomas, lymphomas, and metastatic carcinomas. Papillary carcinoma is characterized on ThinPrep by sheets and papillary clusters of crowded cells with nuclear enlargement and molding, powdery chromatin, irregular nuclear membranes as evidenced by nuclear grooves and intranuclear cytoplasmic inclusions, and small but prominent and often eosinophilic nucleoli.[7] In contrast, medullary carcinoma is characterized by isolated monomorphic plasmacytoid cells that have a high nucleus:cytoplasm ratio, eccentric nuclei and coarsely granular chromatin with or without a prominent nucleolus. Small, inconspicuous granules fill the cytoplasm. Occasionally, the cells appear spindly, but show the same nuclear features. Lymphomas and metastatic carcinomas of the thyroid are much less common. Their cytologic features depend on their type and site of origin, respectively.
Suspicious for malignancy
When the specimen is not hypercellular or most, but not all, of the above described features for papillary and medullary carcinoma or other malignancy are present, it is reported to be suspicious for malignancy. The positive predictive value (malignancy rate on histology) of “suspicious for papillary carcinoma” ranges from 54 to 84% in the literature, depending on whether another less than definitive category, such as indeterminate, is in the scheme. [7, 8, 9, 10] When an indeterminate category is included in addition to the suspicious category, the PPV for the suspicious category is 64% or higher.[7, 9, 10] Since other types of carcinomas in the thyroid are much less common, their positive predictive values of a suspicious diagnosis have not been reported.
Indeterminate for malignancy
This category in our lab includes those specimens that have a predictive value of malignancy on histology less than 50% (mostly less than 30%) and greater than 10%.[6, 7] This includes those specimens that show a few features of papillary carcinoma, but are insufficient for a suspicious diagnosis.[7] Its positive predictive value ranges from 20 to 54% in the literature.[7, 9, 10, 11] These lesions often represent follicular variant of papillary carcinoma. This diagnostic category also includes follicular and Hürthle cell neoplasms, characterized by scant colloid and follicular cells in microfollicles and crowded groups or single Hürthle cells. The positive predictive value of these lesions for a carcinoma varied from 2 to 91% in the literature,[6, 9, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27] probably due to different definitions of these terms and varying criteria in their scheme as well as on histologic diagnosis, but is less than 30% in most but a few reports.[10, 15, 22, 24, 27]
Most probably benign
This category includes those follicular lesions that show a macrofollicular or mixed micro- and macrofollicular pattern with some to abundant colloid in the background. On ThinPrep macrofollicles are seen as sheets of dozens of evenly spaced follicular cells with good distance between nuclei. The significance of single follicular cells on ThinPrep is uncertain as they are seen in an otherwise micro- or macrofollicular pattern. Since the probability for these lesions to show malignancy (follicular variant of papillary carcinoma or follicular carcinoma) on histology is low but not exactly zero, ranging from 0 to 43% in the literature [6, 9, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 24, 25, 26, 27] with all but three[14, 16, 27] less than 6%, we therefore call it most probably benign instead of unequivocally benign.
Negative for malignant cells consistent with Hashimoto’s thyroiditis
We have found that ThinPrep is not optimal for the diagnosis of thyroiditis.[6] Hashimoto’s thyroiditis is characterized by sheets or groups of follicular cells with varying degrees of Hürthle cell changes in a background of varying number of lymphocytes and plasma cells with occasional lymphoid and follicular center aggregates. Since lymphocytic/Hashimoto’s thyroiditis is a common finding in the thyroid, sampling is critical in ruling out other co-existing more significant lesions.
Sub-optimal cellularity but shows features suggestive of …
Since thyroid FNA essentially triages patients for either surgery or follow-up, we would like to have more than adequate cellularity for a definitive diagnosis for this triage. If the cellularity is sub-optimal (but enough to suggest a diagnosis), we would start the report with this qualifier and then follow with any one of the above categories. This category includes those specimens that show cyst contents (macrophages) with very few follicular cells. We do not have a quantitative threshold for this category but rely on a combination of features, including the amount of colloid, cellular arrangement as well cytologic features, to make this decision.
Non-diagnostic
This category is usually reserved for those specimens that show virtually no, or very few, follicular cells.
References
- Cramer H. Fine-needle aspiration cytology of the thyroid: an appraisal. Cancer 2000; 90(6):325-9.
- Mazzaferri EL. Management of a solitary thyroid nodule [see comments]. N Engl J Med 1993; 328(8):553-9.
- Biscotti CV, Hollow JA, Toddy SM, Easley KA. ThinPrep versus conventional smear cytologic preparations in the analysis of thyroid fine-needle aspiration specimens. Am J Clin Pathol 1995; 104(2):150-3.
- Frost AR, Sidawy MK, Ferfelli M, et al. Utility of thin-layer preparations in thyroid fine-needle aspiration: diagnostic accuracy, cytomorphology, and optimal sample preparation. Cancer 1998; 84(1):17-25.
- Afify AM, Liu J, Al-Khafaji BM. Cytologic artifacts and pitfalls of thyroid fine-needle aspiration using ThinPrep: a comparative retrospective review. Cancer 2001; 93(3):179-86.
- Tulecke MA, Wang HH. ThinPrep for cytologic evaluation of follicular thyroid lesions: correlation with histologic findings. Diagn Cytopathol 2004; 30(1):7-13.
- Zhang Y, Fraser JL, Wang HH. Morphologic predictors of papillary carcinoma on fine-needle aspiration of thyroid with ThinPrep preparations. Diagn Cytopathol 2001; 24(6):378-83.
- Chen H, Zeiger MA, Clark DP, Westra WH, Udelsman R. Papillary carcinoma of the thyroid: can operative management be based solely on fine-needle aspiration? J Am Coll Surg 1997; 184(6):605-10.
- Renshaw AA. Accuracy of thyroid fine-needle aspiration using receiver operator characteristic curves. Am J Clin Pathol 2001; 116(4):477-82.
- Goldstein RE, Netterville JL, Burkey B, Johnson JE. Implications of follicular neoplasms, atypia, and lesions suspicious for malignancy diagnosed by fine-needle aspiration of thyroid nodules. Ann Surg 2002; 235(5):656-62; discussion 662-4.
- Renshaw AA. Focal features of papillary carcinoma of the thyroid in fine-needle aspiration material are strongly associated with papillary carcinoma at resection. Am J Clin Pathol 2002; 118(2):208-10.
- Al-Rikabi AC, Al-Omran M, Cheema M, El-Khwsky F, Al-Nuaim A. Pattern of thyroid lesions and role of fine needle aspiration cytology (FNA) in the management of thyroid enlargement: a retrospective study from a teaching hospital in Riyadh. Apmis 1998; 106(11):1069-74.
- Baloch ZW, Tam D, Langer J, Mandel S, LiVolsi VA, Gupta PK. Ultrasound-guided fine-needle aspiration biopsy of the thyroid: role of on-site assessment and multiple cytologic preparations. Diagn Cytopathol 2000; 23(6):425-9.
- Boyd LA, Earnhardt RC, Dunn JT, Frierson HF, Hanks JB. Preoperative evaluation and predictive value of fine-needle aspiration and frozen section of thyroid nodules. J Am Coll Surg 1998; 187(5):494-502.
- Hawkins F, Bellido D, Bernal C, et al. Fine needle aspiration biopsy in the diagnosis of thyroid cancer and thyroid disease. Cancer 1987; 59(6):1206-9.
- Ko HM, Jhu IK, Yang SH, et al. Clinicopathologic analysis of fine needle aspiration cytology of the thyroid. A review of 1,613 cases and correlation with histopathologic diagnoses. Acta Cytol 2003; 47(5):727-32.
- Mandreker SR, Nadkarni NS, Pinto RG, Menezes S. Role of fine needle aspiration cytology as the initial modality in the investigation of thyroid lesions. Acta Cytol 1995; 39(5):898-904.
- Miller JM, Kini SR, Hamburger JI. The diagnosis of malignant follicular neoplasms of the thyroid by needle biopsy. Cancer 1985; 55(12):2812-7.
- Morgan JL, Serpell JW, Cheng MS. Fine-needle aspiration cytology of thyroid nodules: how useful is it? ANZ J Surg 2003; 73(7):480-3.
- Prinz RA, O’Morchoe PJ, Barbato AL, et al. Fine needle aspiration biopsy of thyroid nodules. Ann Surg 1983; 198(1):70-3.
- Ravetto C, Colombo L, Dottorini ME. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: a retrospective study in 37,895 patients. Cancer 2000; 90(6):357-63.
- Nam-Goong IS, Kim HY, Gong G, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004; 60(1):21-8.
- Silverman JF, West RL, Larkin EW, et al. The role of fine-needle aspiration biopsy in the rapid diagnosis and management of thyroid neoplasm. Cancer 1986; 57(6):1164-70.
- La Rosa GL, Belfiore A, Giuffrida D, et al. Evaluation of the fine needle aspiration biopsy in the preoperative selection of cold thyroid nodules. Cancer 1991; 67(8):2137-41.
- DeMay RM. Follicular lesions of the thyroid. W(h)ither follicular carcinoma? Am J Clin Pathol 2000; 114(5):681-3.
- Mateša N, Tabain I, Dabelic N, Petric V, Kusic Z. Diagnostic relevance of fine needle aspiration cytology for follicular lesions of the thyroid: retrospective study. Croat Med J 2002; 43(5):606-9.
- Blansfield JA, Sack MJ, Kukora JS. Recent experience with preoperative fine-needle aspiration biopsy of thyroid nodules in a community hospital. Arch Surg 2002; 137(7):818-21.

Positive for Papillary Carcinoma
Reminder: You may click on any slide image
for an enlarged view.
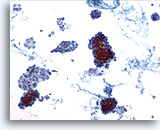
Figure 1
Papillary carcinoma.
The first criterion of a definitive diagnosis of papillary carcinoma is hypercellularity with many cohesive sheets and clusters of follicular cells at low magnification as shown in this figure. 10x
Figure 1
Papillary carcinoma.
The first criterion of a definitive diagnosis of papillary carcinoma is hypercellularity with many cohesive sheets and clusters of follicular cells at low magnification as shown in this figure. 10x
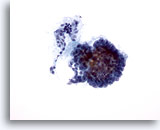
Figure 2
Papillary carcinoma.
Papillary architecture as seen in this figure is not required for a definitive diagnosis of papillary carcinoma. 20X
Figure 2
Papillary carcinoma.
Papillary architecture as seen in this figure is not required for a definitive diagnosis of papillary carcinoma.
20X
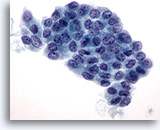
Figure 3
Papillary carcinoma.
On ThinPrep preparations papillary carcinomas are often present as sheets of enlarged follicular cells with irregular nuclear membranes, and small but prominent and often eosinophilic nucleoli. 60X
Figure 3
Papillary carcinoma.
On ThinPrep preparations papillary carcinomas are often present as sheets of enlarged follicular cells with irregular nuclear membranes, and small but prominent and often eosinophilic nucleoli.
60X
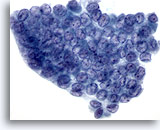
Figure 4
Papillary carcinoma.
Nuclear molding, crowding and overlapping, as a result of marked nuclear enlargement, as well as powdery chromatin, are illustrated in this figure along with nuclear grooves and intranuclear cytoplasmic inclusions. 60X
Figure 4
Papillary carcinoma.
Nuclear molding, crowding and overlapping, as a result of marked nuclear enlargement, as well as powdery chromatin, are illustrated in this figure along with nuclear grooves and intranuclear cytoplasmic inclusions.
60X
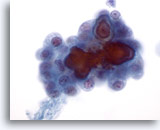
Figure 5
Papillary carcinoma. Although features demonstrated in figures 1-4 are sufficient for a definitive diagnosis of papillary carcinoma, it is re-assuring to see psammoma bodies as shown in this figure. Psammoma bodies are rarely seen and are not required for a definitive diagnosis of papillary carcinoma. 60x
Figure 5
Papillary carcinoma.
Although features demonstrated in figures 1-4 are sufficient for a definitive diagnosis of papillary carcinoma, it is re-assuring to see psammoma bodies as shown in this figure. Psammoma bodies are rarely seen and are not required for a definitive diagnosis of papillary carcinoma.
60x
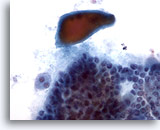
Figure 6
Papillary carcinoma.
Although non-specific, dense colloid as seen in this figure, is sometimes seen in papillary carcinoma. 40x
Figure 6
Papillary carcinoma.
Although non-specific, dense colloid as seen in this figure, is sometimes seen in papillary carcinoma.
40x
Suspicious for Papillary Carcinoma
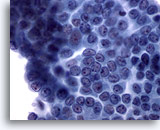
Figure 7: Papillary carcinoma. Many, but not all, of the features of papillary carcinoma, such as powdery chromatin, nuclear grooves, and small prominent nucleoli, are present in this sheet of cells. Intranuclear cytoplasmic inclusions are absent. In addition, nuclei are enlarged but not to the degree of molding and crowding. Sixty-five to 90% of such cases are confirmed to be papillary carcinoma on histology. For the sake of the remaining 10-35% of the cases this specimen probably is best reported as suspicious for papillary carcinoma. 60x
Figure 7
Papillary carcinoma.
Many, but not all, of the features of papillary carcinoma, such as powdery chromatin, nuclear grooves, and small prominent nucleoli, are present in this sheet of cells. Intranuclear cytoplasmic inclusions are absent. In addition, nuclei are enlarged but not to the degree of molding and crowding. Sixty-five to 90% of such cases are confirmed to be papillary carcinoma on histology. For the sake of the remaining 10-35% of the cases this specimen probably is best reported as suspicious for papillary carcinoma.
60x
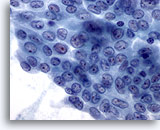
Figure 8
Papillary carcinoma. Similar to figure 7, this sheet of cells have most, but not all, of the features of a papillary carcinoma. It may be debatable whether this should be reported as positive or suspicious for papillary carcinoma. Both are correct. Of course, cells found on the rest of the specimen would also contribute to the final report. 60X
Figure 8
Papillary carcinoma.
Similar to figure 7, this sheet of cells have most, but not all, of the features of a papillary carcinoma. It may be debatable whether this should be reported as positive or suspicious for papillary carcinoma. Both are correct. Of course, cells found on the rest of the specimen would also contribute to the final report.
60X
Indeterminate for Papillary Carcinoma – Papillary Carcinoma with Cystic Change
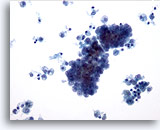
Figure 9
Papillary carcinoma.
Cystic change characterized by background macrophages as seen in this figure is common in papillary carcinoma. 20x
Figure 9
Papillary carcinoma.
Cystic change characterized by background macrophages as seen in this figure is common in papillary carcinoma.
20x
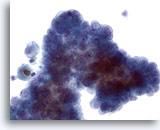
Figure 10
Papillary carcinoma. When papillary carcinoma becomes cystic, its characteristic nuclear features somehow are less apparent. Small but prominent nucleoli with powdery chromatin are seen in this crowded group of cells. However, nuclear enlargement and irregular membranes are not obvious. 40x
Figure 10
Papillary carcinoma.
When papillary carcinoma becomes cystic, its characteristic nuclear features somehow are less apparent. Small but prominent nucleoli with powdery chromatin are seen in this crowded group of cells. However, nuclear enlargement and irregular membranes are not obvious.
40x
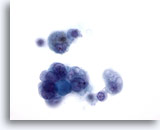
Figure 11: Papillary carcinoma. This field is from the same specimen as Figure 10. The nuclear features of this histology-confirmed papillary carcinoma are even less apparent for papillary carcinoma in this field. Since not all lesions with a few nuclear features of papillary carcinoma, with or without cystic change, are papillary carcinoma, it is best to report this kind of specimens as “indeterminate” for papillary carcinoma. In the literature 20% – 54% of such cases are confirmed to be papillary carcinoma on histology. 60x
Figure 11
Papillary carcinoma.
This field is from the same specimen as Figure 10. The nuclear features of this histology-confirmed papillary carcinoma are even less apparent for papillary carcinoma in this field. Since not all lesions with a few nuclear features of papillary carcinoma, with or without cystic change, are papillary carcinoma, it is best to report this kind of specimens as “indeterminate” for papillary carcinoma. In the literature 20% – 54% of such cases are confirmed to be papillary carcinoma on histology.
60x
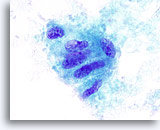
Figure 12
Indeterminate for papillary carcinoma, Hyalinizing trabecular adenoma. This group of follicular cells show nuclear irregularities, small prominent nucleoli and a probable inclusion. This FNA was reported as “indeterminate for papillary carcinoma.” The thyroidectomy specimen showed a hyalinizing trabecular adenoma. 100x
Figure 12
Indeterminate for papillary carcinoma, Hyalinizing trabecular adenoma.
This group of follicular cells show nuclear irregularities, small prominent nucleoli and a probable inclusion. This FNA was reported as “indeterminate for papillary carcinoma.” The thyroidectomy specimen showed a hyalinizing trabecular adenoma.
100x
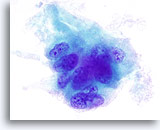
Figure 13
Indeterminate for papillary carcinoma, Hyalinizing trabecular adenoma.
As in Figure 12, nuclear grooves and small prominent nucleoli are noted. The green amorphous material surrounding this group of cells is in fact collagen when compared to the histology of the lesion. 100x
Figure 13
Indeterminate for papillary carcinoma, Hyalinizing trabecular adenoma.
As in Figure 12, nuclear grooves and small prominent nucleoli are noted. The green amorphous material surrounding this group of cells is in fact collagen when compared to the histology of the lesion.
100x
Positive for Medullary Carcinoma

Figure 14
Medullary carcinoma.
Medullary carcinoma typically presents on FNA as loose groups of and single monomorphic plasmacytoid cells. A fragment of amyloid is seen in this field. 20x
Figure 14
Medullary carcinoma.
Medullary carcinoma typically presents on FNA as loose groups of and single monomorphic plasmacytoid cells. A fragment of amyloid is seen in this field.
20x
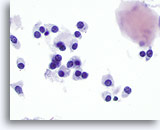
Figure 15
Medullary carcinoma.
On high magnification, the tumor cells are round, oval, polygonal, or spindle in shape with similar appearing nuclei. A fragment of amyloid is at the right upper corner of the figure. 60x
Figure 15
Medullary carcinoma.
On high magnification, the tumor cells are round, oval, polygonal, or spindle in shape with similar appearing nuclei. A fragment of amyloid is at the right upper corner of the figure.
60x
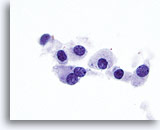
Figure 16
Medullary carcinoma.
On even higher magnification, finely granular cytoplasm and coarsely granular chromatin are appreciated. Occasional prominent nucleoli are seen as well as occasional bi-nucleation which is characteristic of medullary carcinoma. 100x
Figure 16
Medullary carcinoma.
On even higher magnification, finely granular cytoplasm and coarsely granular chromatin are appreciated. Occasional prominent nucleoli are seen as well as occasional bi-nucleation which is characteristic of medullary carcinoma.
100x
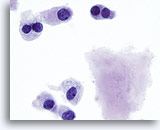
Figure 17
Medullary carcinoma.
More bi-nucleated cells are seen with amyloid. 100x
Figure 17
Medullary carcinoma.
More bi-nucleated cells are seen with amyloid.
100x
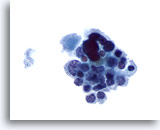
Figure 18
Medullary carcinoma. A loose group of mostly small uniform cells with occasional large atypical ones is seen in this figure. The small cells have eccentric nuclei and finely granular cytoplasm. This and the following figures illustrate an unusual presentation of medullary carcinoma on FNA. 60x
Figure 18
Medullary carcinoma.
A loose group of mostly small uniform cells with occasional large atypical ones is seen in this figure. The small cells have eccentric nuclei and finely granular cytoplasm. This and the following figures illustrate an unusual presentation of medullary carcinoma on FNA.
60x
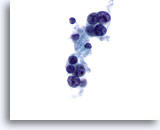
Figure 19
Medullary carcinoma.
Occasionally, medullary carcinoma shows prominent nucleoli as seen in this figure. Most of the cells in this figure have lost their cytoplasm. 60x
Figure 19
Medullary carcinoma.
Occasionally, medullary carcinoma shows prominent nucleoli as seen in this figure. Most of the cells in this figure have lost their cytoplasm.
60x
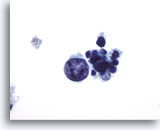
Figure 20
Medullary carcinoma.
One very large, atypical, but degenerated tumor cell is seen in this field with several small uniform degenerated tumor cells. 60x
Figure 20
Medullary carcinoma.
One very large, atypical, but degenerated tumor cell is seen in this field with several small uniform degenerated tumor cells.
60x
Positive for Anaplastic Thyroid Carcinoma
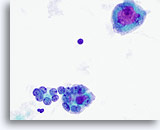
Figure 21
Anaplastic carcinoma. Two microfollicles are seen in the lower half of the figure. They are composed of variably enlarged atypical follicular cells with prominent nucleoli in some. An osteoclast-like giant cell is in the upper half with large and prominent nucleoli. 60x
Figure 21
Anaplastic carcinoma.
Two microfollicles are seen in the lower half of the figure. They are composed of variably enlarged atypical follicular cells with prominent nucleoli in some. An osteoclast-like giant cell is in the upper half with large and prominent nucleoli.
60x
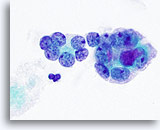
Figure 22
Anaplastic carcinoma.
A higher magnification of the microfollicles in Figure 21. 100x
Figure 22
Anaplastic carcinoma.
A higher magnification of the microfollicles in Figure 21.
100x
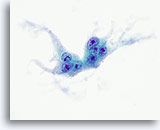
Figure 23
Anaplastic carcinoma.
Another osteoclast-like giant cell. 60x
Figure 23
Anaplastic carcinoma.
Another osteoclast-like giant cell.
60x
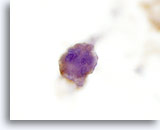
Figure 24
Anaplastic carcinoma.
The tumor cells are positive for thyroglobulin by immunocytochemistry confirming their thyroid origin. 60x
Figure 24
Anaplastic carcinoma.
The tumor cells are positive for thyroglobulin by immunocytochemistry confirming their thyroid origin.
60x
Indeterminate for Malignancy – Hürthle Cell Neoplasm
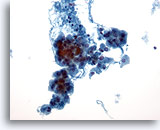
Figure 25
Hürthle cell neoplasm.
Hürthle cell neoplasms on cytology are characterized by a cellular specimen with many dyshesive clusters of and single Hürthle cells on low magnification. 20x
Figure 25
Hürthle cell neoplasm.
Hürthle cell neoplasms on cytology are characterized by a cellular specimen with many dyshesive clusters of and single Hürthle cells on low magnification.
20x
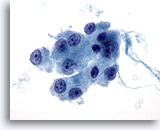
Figure 26
Hürthle cell neoplasm.
On high magnification, moderate to abundant granular cytoplasm and central prominent nucleoli characterize Hürthle cells. 60x
Figure 26
Hürthle cell neoplasm.
On high magnification, moderate to abundant granular cytoplasm and central prominent nucleoli characterize Hürthle cells.
60x
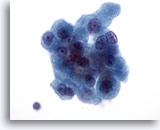
Figure 27
Hürthle cell neoplasm.
Bi-nucleation, as seen in the right upper corner and the left lower corner of this cluster of cells, is another characteristic of Hürthle cells. 60x
Figure 27
Hürthle cell neoplasm.
Bi-nucleation, as seen in the right upper corner and the left lower corner of this cluster of cells, is another characteristic of Hürthle cells.
60x
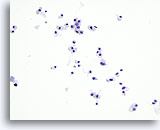
Figure 28
Hürthle cell neoplasm.
Similar to medullary carcinoma, Hürthle cell neoplasm presents on FNA as many loose groups of and isolated tumor cells. The cytoplasm in Hürthle cell neoplasm is more abundant than that in medullary carcinoma. 20x
Figure 28
Hürthle cell neoplasm.
Similar to medullary carcinoma, Hürthle cell neoplasm presents on FNA as many loose groups of and isolated tumor cells. The cytoplasm in Hürthle cell neoplasm is more abundant than that in medullary carcinoma.
20x
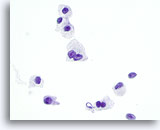
Figure 29
Hürthle cell neoplasm.
Frequent bi-nucleation, as seen in this figure, is another feature of Hürthle cell neoplasm that is shared by medullary carcinoma. 60x
Figure 29
Hürthle cell neoplasm.
Frequent bi-nucleation, as seen in this figure, is another feature of Hürthle cell neoplasm that is shared by medullary carcinoma.
60x
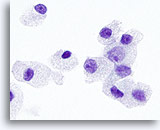
Figure 30
Hürthle cell neoplasm.
On high magnification, the cytoplasmic granules and the nucleoli are larger and more prominent in Hürthle cell neoplasms than those in medullary carcinomas. 100x
Figure 30
Hürthle cell neoplasm.
On high magnification, the cytoplasmic granules and the nucleoli are larger and more prominent in Hürthle cell neoplasms than those in medullary carcinomas.
100x
Indeterminate for Malignancy – Microfollicular Neoplasm With Hürthle Cell Features
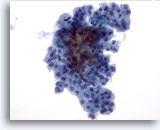
Figure 31
Microfollicular neoplasm, With Hürthle cell change.
Crowded groups of follicular cells are seen in the specimen with no colloid in the background. 40x
Figure 31
Microfollicular neoplasm, With Hürthle cell change.
Crowded groups of follicular cells are seen in the specimen with no colloid in the background.
40x
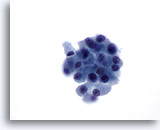
Figure 32
Microfollicular neoplasm, With Hürthle cell change.
On higher magnification, the follicular cells are somewhat dyshesive with a fair amount of granular cytoplasm. However, not all the cells have large prominent nucleoli. 60x
Figure 32
Microfollicular neoplasm, With Hürthle cell change.
On higher magnification, the follicular cells are somewhat dyshesive with a fair amount of granular cytoplasm. However, not all the cells have large prominent nucleoli.
60x
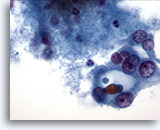
Figure 33
Microfollicular neoplasm, With Hürthle cell change.
The Hürthle cells in the right field of this figure are apparently forming a microfollicle. 60x
Figure 33
Microfollicular neoplasm, With Hürthle cell change.
The Hürthle cells in the right field of this figure are apparently forming a microfollicle.
60x
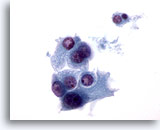
Figure 34
Microfollicular neoplasm, With Hürthle cell change.
Elsewhere in the same specimen as Figure 33, the dyshesive nature of these Hürthle cells is more obvious. 60x
Figure 34
Microfollicular neoplasm, With Hürthle cell change.
Elsewhere in the same specimen as Figure 33, the dyshesive nature of these Hürthle cells is more obvious.
60x
Indeterminate for Malignancy – Microfollicular Neoplasm
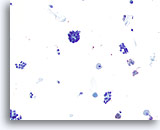
Figure 35
Microfollicular neoplasm.
Scattered crowded groups/microfollicles are seen on this relatively low magnification with some macrophages in the background. 20x
Figure 35
Microfollicular neoplasm.
Scattered crowded groups/microfollicles are seen on this relatively low magnification with some macrophages in the background.
20x
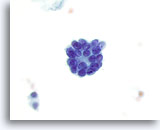
Figure 36
Microfollicular neoplasm.
A high magnification view of a microfollicle. 60x
Figure 36
Microfollicular neoplasm.
A high magnification view of a microfollicle.
60x
Most Probably Benign – Consistent with a Mixed Micro- and Macrofollicular Lesion
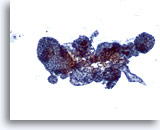
Figure 37
Macrofollicular lesion.
Several variably-sized follicles are present. 10x
Figure 37
Macrofollicular lesion.
Several variably-sized follicles are present.
10x
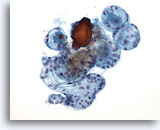
Figure 38
Macrofollicular lesion.
A higher magnification than figure 37 shows more variably-sized intact follicles. 20x
Figure 38
Macrofollicular lesion.
A higher magnification than figure 37 shows more variably-sized intact follicles.
20x
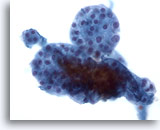
Figure 39
Macrofollicular lesion.
A still higher magnification shows a group of small follicles. 40x
Figure 39
Macrofollicular lesion.
A still higher magnification shows a group of small follicles.
40x
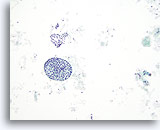
Figure 40
Macrofollicular lesion.
A macrofollicle and a sheet of follicular cells are seen in this figure. Whether in follicles or in sheets, macrofollicles are characterized by widely space follicular cells. 10x
Figure 40
Macrofollicular lesion.
A macrofollicle and a sheet of follicular cells are seen in this figure. Whether in follicles or in sheets, macrofollicles are characterized by widely space follicular cells.
10x
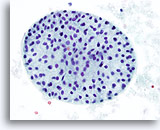
Figure 41
Macrofollicular lesion.
A high magnification of the macrofollicle in Figure 40. 40x
Figure 41
Macrofollicular lesion.
A high magnification of the macrofollicle in Figure 40.
40x

Figure 42
Macrofollicular lesion.
More often macrofollicles are presented as sheets of follicular cells as seen in this figure. 20x
Figure 42
Macrofollicular lesion.
More often macrofollicles are presented as sheets of follicular cells as seen in this figure.
20x
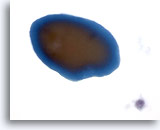
Figure 43
Macrofollicular lesion.
Colloid typically appears as a fragment of homogeneous amorphous material with a sharp edge and two tones of color on ThinPrep. 60x
Figure 43
Macrofollicular lesion.
Colloid typically appears as a fragment of homogeneous amorphous material with a sharp edge and two tones of color on ThinPrep.
60x
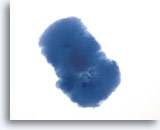
Figure 44
Macrofollicular lesion.
Colloid can also has a slightly irregular texture on ThinPrep. 60x
Figure 44
Macrofollicular lesion.
Colloid can also ha
s a slightly irregular texture on ThinPrep.
60x
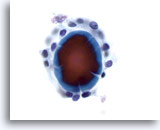
Figure 45
Macrofollicular lesion.
The fragment of colloid in the center of a follicle shows cracking artifacts. 60x
Figure 45
Macrofollicular lesion.
The fragment of colloid in the center of a follicle shows cracking artifacts.
60x
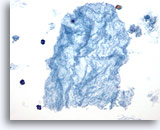
Figure 46
Macrofollicular lesion.
This tissue-paper-like material has been shown to be strongly associated with the amount of colloid on thyroidectomy specimen and most likely represents watery colloid. 40x
Figure 46
Macrofollicular lesion.
This tissue-paper-like material has been shown to be strongly associated with the amount of colloid on thyroidectomy specimen and most likely represents watery colloid.
40x
Negative for Malignant Cells – Consistent with Hashimoto’s Thyroiditis

Figure 47
Hashimoto’s thyroiditis.
Many mononuclear cells with scant cytoplasm and a lymphoid follicle are seen in this figure. 20x
Figure 47
Hashimoto’s thyroiditis.
Many mononuclear cells with scant cytoplasm and a lymphoid follicle are seen in this figure.
20x
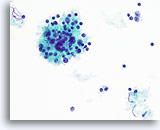
Figure 48
Hashimoto’s thyroiditis.
A higher magnification shows the mononuclear cells and lymphoid follicle with lymphoid cells at various stages of maturation. 40x
Figure 48
Hashimoto’s thyroiditis.
A higher magnification shows the mononuclear cells and lymphoid follicle with lymphoid cells at various stages of maturation.
40x
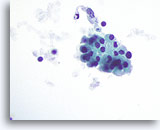
Figure 49
Hashimoto’s thyroiditis.
A cohesive group of Hürthle cells with granular cytoplasm is seen elsewhere on the specimen. 60x
Figure 49
Hashimoto’s thyroiditis.
A cohesive group of Hürthle cells with granular cytoplasm is seen elsewhere on the specimen.
60x
Back to Top

















































